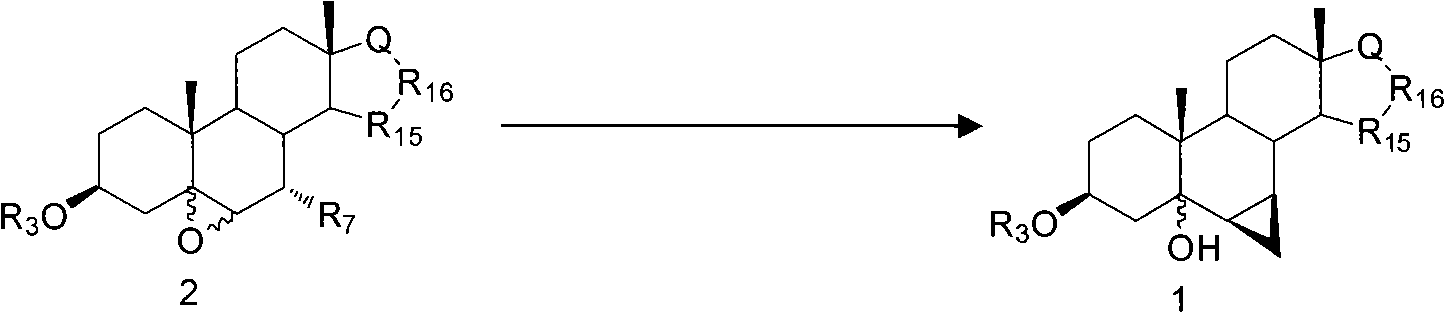
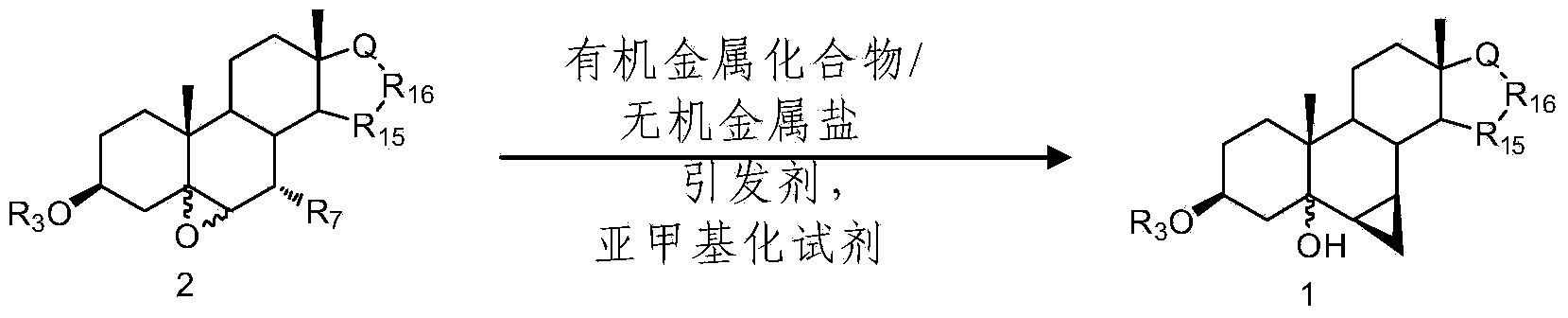
Method for constructing sterides-6beta, 7beta-methylene structure by cascade reaction
A tandem reaction and methylene technology, which is applied in the direction of chemical instruments and methods, steroids, steroids, etc., can solve the problems of expensive reaction reagents, many reaction steps, long reaction steps, etc., and achieve cheap raw materials and short reaction steps. The effect of less and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of compound 3β-acetoxy-6β,7β; 15β,16β-dimethylene-5α-androst-5-ol-17-one:
[0023] At room temperature, to ether (100mL ) solution, add cuprous iodide (762mg, 4.0mmol) and montmorillonite K-10 (300mg), stir for 0.5h to 1h, then add Zn-Cu preparation (2.8g), diiodomethane (3.2mL). Raise the temperature to 65°C-70°C, and keep it warm for 4 hours. After the reaction was completed, cool down to room temperature, add dichloromethane (100mL) to dilute, wash with saturated ammonium chloride aqueous solution and water successively, dry over anhydrous magnesium sulfate, separate by suction filtration, and remove the solvent by extraction under reduced pressure to obtain an oily substance. Column separation (V 石油醚 / V 乙酸乙酯 =1:2), remove the solvent under reduced pressure, and obtain the target compound 3β-acetoxy-6β, 7β after vacuum drying at 40°C; 15β, 16β-dimethylene-5α-androst-5-ol-17-one , off-white powder, 834mg (83%, 2.2mmol).
[0024] In this example, the sta...
Embodiment 2
[0026] Synthesis of compound 3β-acetoxy-6β,7β; 15β,16β-dimethylene-5α-androst-5-ol-17-one:
[0027] At room temperature, diethyl ether (100mL ) solution was added cuprous iodide (762mg, 4.0mmol), lithium bromide (300mg) and lithium carbonate (300mg), stirred for 0.5h and added Zn-Cu preparation (2.8g), diiodomethane (3.2mL). Raise the temperature to 65°C-70°C, and keep it warm for 4 hours. After the reaction was completed, cool down to room temperature, add dichloromethane (100mL) to dilute, wash with saturated ammonium chloride aqueous solution and water successively, dry over anhydrous magnesium sulfate, separate by suction filtration, and remove the solvent by extraction under reduced pressure to obtain an oily substance. Column separation (V 石油醚 / V 甲醇 =1:2), remove the solvent under reduced pressure, and obtain the target compound 3β-acetoxy-6β, 7β after vacuum drying at 40°C; 15β, 16β-dimethylene-5α-androst-5-ol-17-one , off-white powder, 894mg (89%, 2.4mmol).
[002...
Embodiment 3
[0030] Synthesis of compound 3β-benzoyloxy-6β,7β; 15β,16β-dimethylene-17α-pregna-5α-ol-21,17-carboxylide:
[0031]At room temperature, 3β, 7α-dibenzoyloxy-5α, 6α-epoxy-15β, 16β-methylene-17α-pregna-21, 17-carboxylide (1.0g, 1.6mmol) in ether (100mL) solution, add cuprous iodide (462mg) and titanocene chloride (100mg), stir for 0.5h to 1h, then add Zn-Cu preparation (1.8g), elemental iodine (20mg), Diiodomethane (1.8 mL). Raise the temperature to 75°C-80°C, and keep it warm for 5 hours. After the reaction was completed, cool down to room temperature, add dichloromethane (100mL) to dilute, wash with saturated ammonium chloride aqueous solution and water successively, dry over anhydrous magnesium sulfate, separate by suction filtration, and remove the solvent by extraction under reduced pressure to obtain an oily substance. Column separation (V 石油醚 / V 乙酸乙酯 =1:2), the solvent was removed under reduced pressure, and the target compound 3β-benzoyloxy-6β, 7β was obtained after va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com