Ritodrine hydrochloride preparation method
A technology for ritodrine hydrochloride and preparation process, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., can solve problems such as high cost, poor product quality, complicated post-processing, etc. The effect of production efficiency and yield, shortened process steps, and short cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
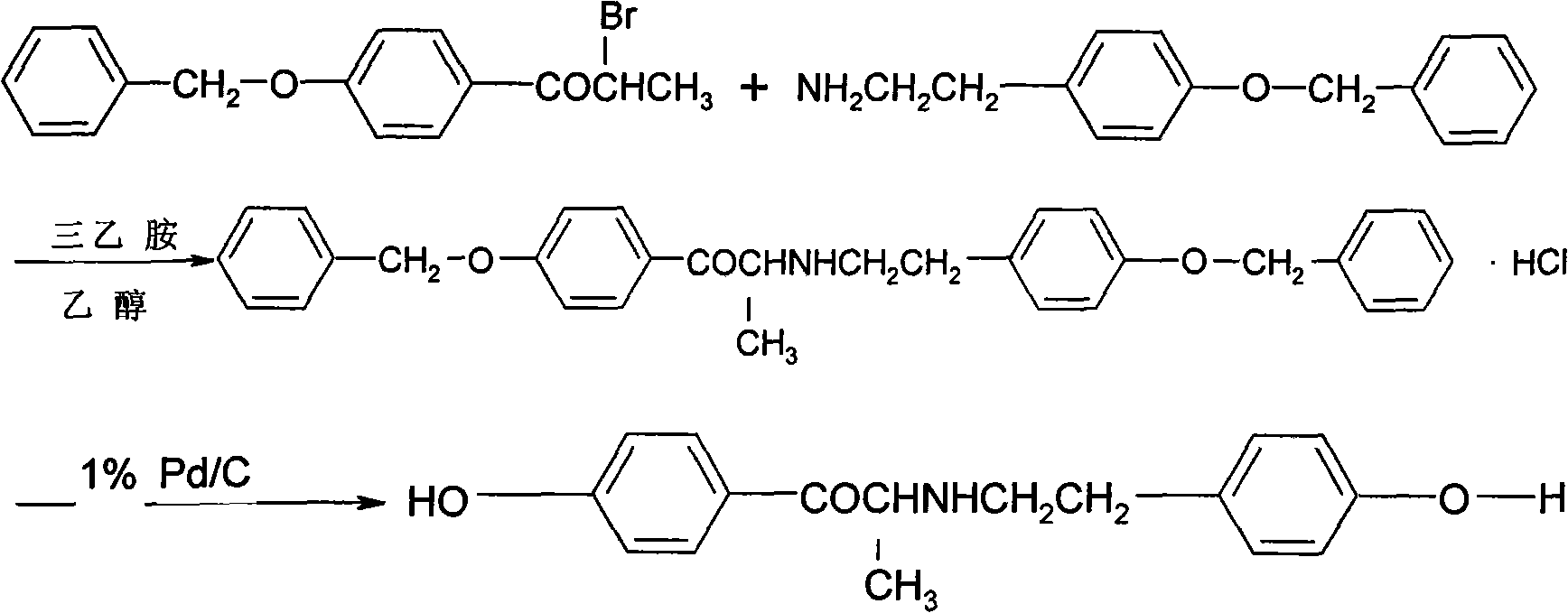
Examples
Embodiment 1
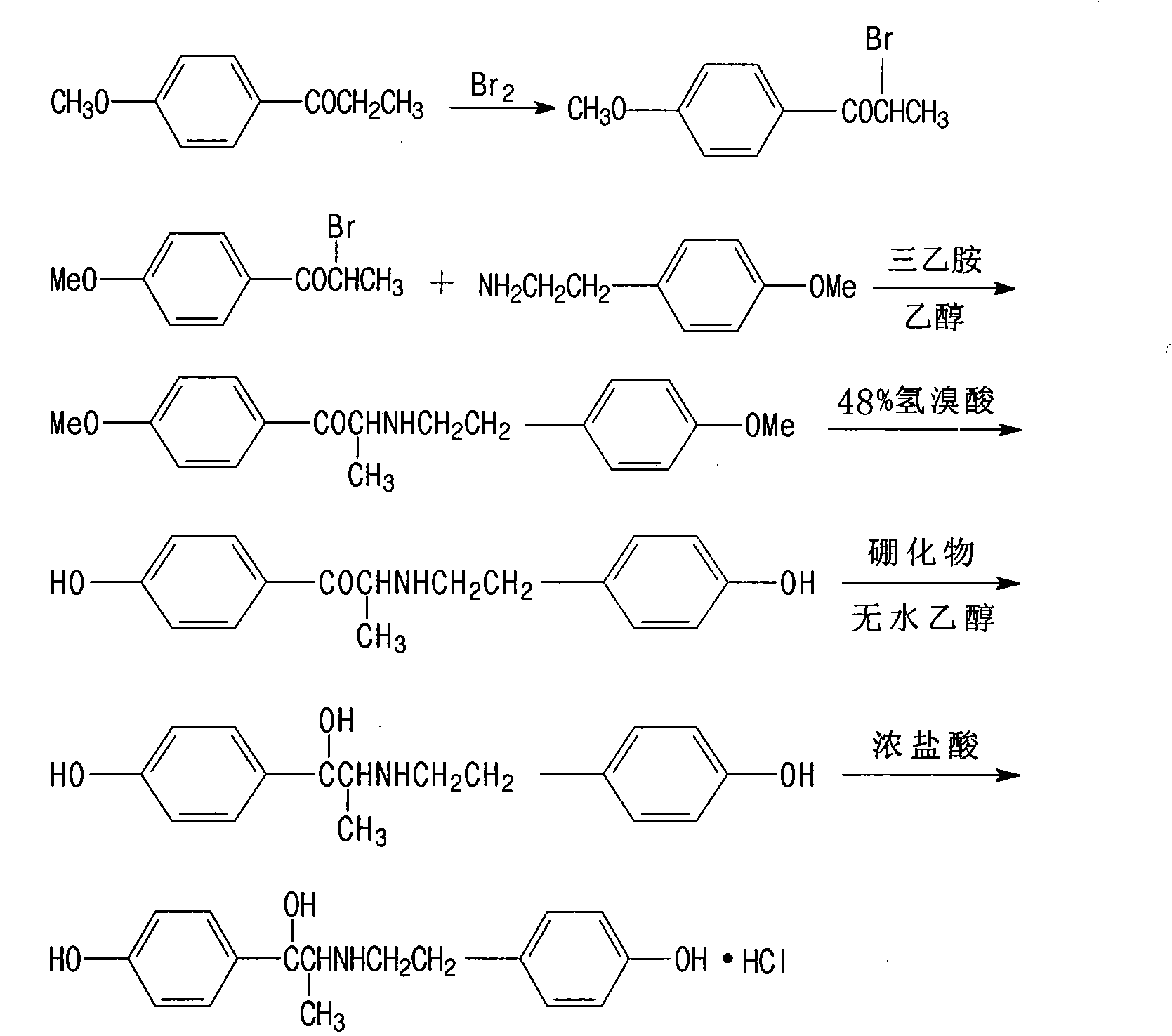
[0034] According to the preparation method of the ritodrine hydrochloride of the present embodiment, it comprises the following steps successively:
[0035] (1) Dissolve 4-methoxypropiophenone in acetone (add a small amount of aluminum trichloride), slowly add bromine under stirring at 5°C, stir for 1.5 hours, evaporate the solvent; add p-methoxypropiophenone to the residue Ethanol solution of phenethylamine and triethylamine, reflux reaction; after the reaction, mix with 48% hydrobromic acid, then reflux reaction for 5h~8h, adjust the pH value with saturated sodium carbonate, extract the reaction solution with dichloromethane; evaporate Dichloromethane, add absolute ethanol and sodium borohydride to the residue, and reflux for 1.5h to 2h; distill off the ethanol, add an appropriate amount of water, extract with dichloromethane, distill off the dichloromethane to obtain white solid ritodrine;
[0036] (2) Add ritodrine to ethanol, slowly add concentrated hydrochloric acid drop...
Embodiment 2
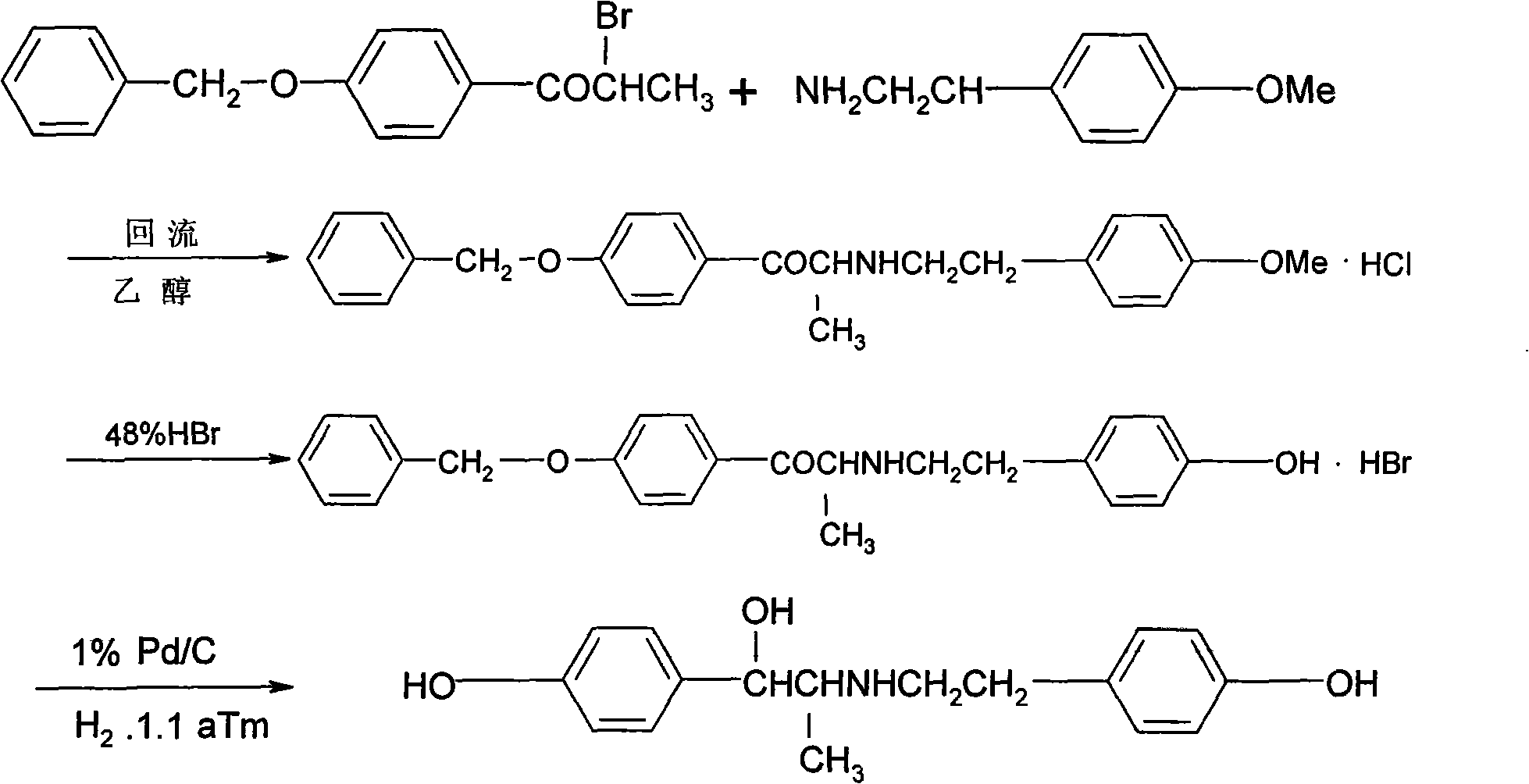
[0038] According to the preparation method of the ritodrine hydrochloride of the present embodiment, it comprises the following steps successively:
[0039] (1) Dissolve 4-methoxypropiophenone in ether (add a small amount of aluminum trichloride), slowly add bromine under stirring at 5°C, stir and react for 2 hours, evaporate the solvent; add p-methoxyl to the residue The ethanol solution of phenethylamine and triethylamine, reflux reaction; after the reaction is completed, mix with 48% hydrobromic acid, and then reflux reaction for 5h-8h, adjust the pH value with saturated sodium bicarbonate solution, and extract the reaction solution with dichloromethane; Evaporate dichloromethane, add absolute ethanol and potassium borohydride to the residue, and reflux for 1.5h to 2h; distill off ethanol, add an appropriate amount of water, extract with dichloromethane, distill off dichloromethane to obtain white solid ritodrine;
[0040] (2) Add ritodrine to ethanol, slowly add concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com