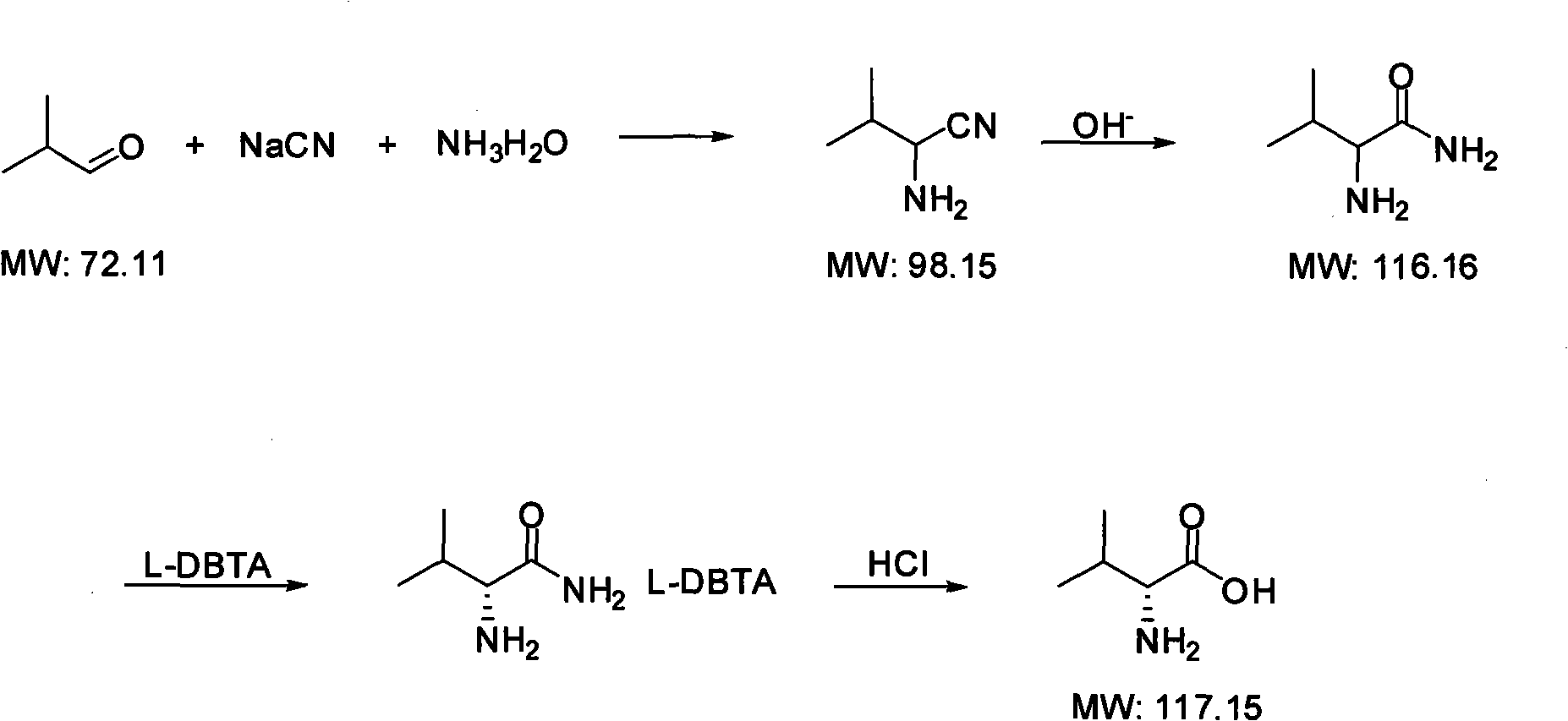
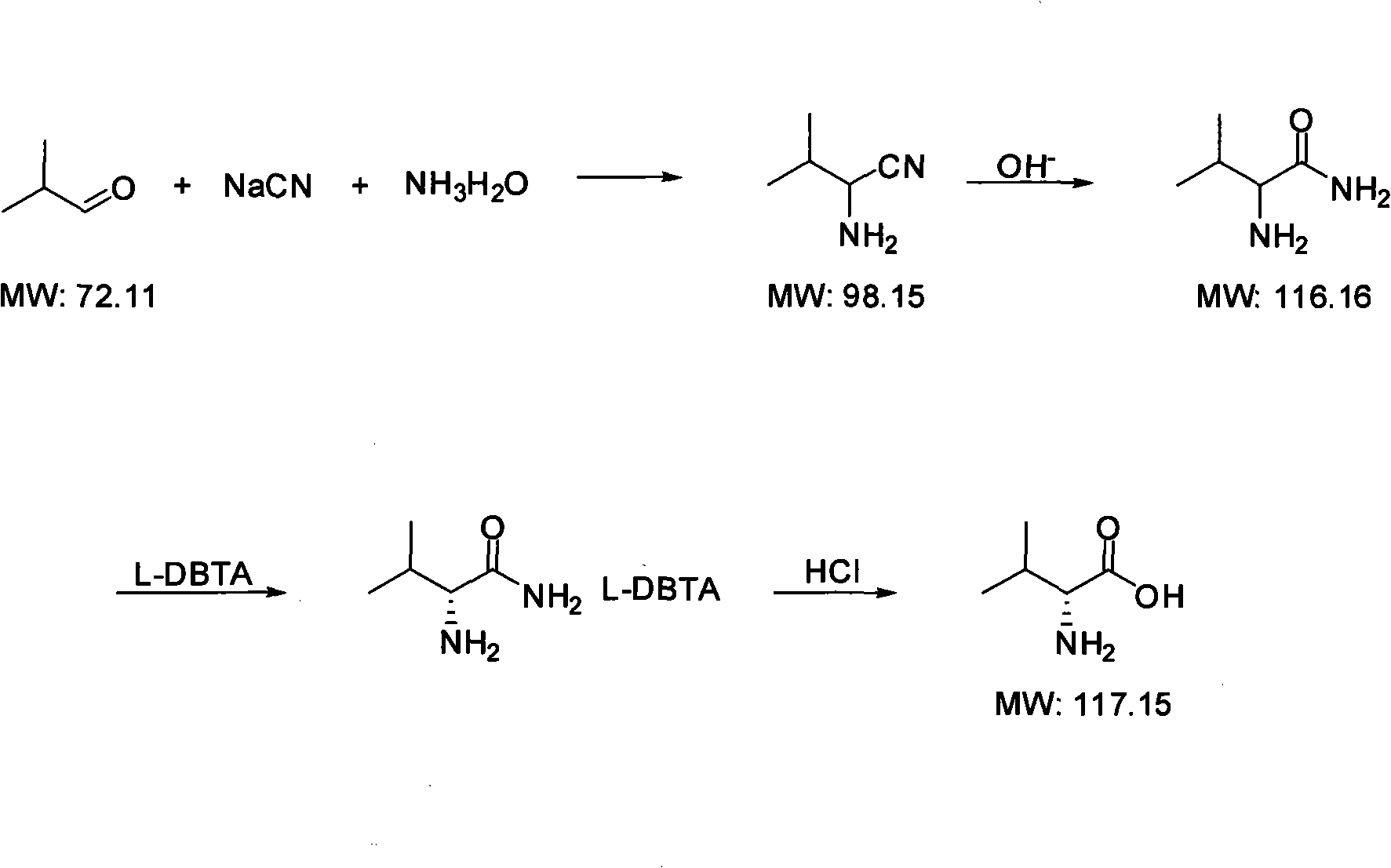
Method for synthesizing D-valine
A synthetic method, valine technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve production limitations, difficulty in controlling the acidity of the split system, split yield and unstable product quality, etc. problems, to achieve the effect of low equipment requirements, less solvent and catalyst consumption, simple and safe unit operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1.1 Preparation of 2-amino-3-methylbutyronitrile:
[0030] In a 1000ml flask, add 200g of 30% sodium cyanide aqueous solution, 86.4g of ammonium chloride, and 111g of 25% ammonia water, stir to dissolve the solid, cool to 0°C, add 100.4g of isobutyraldehyde dropwise, after the dropwise addition is complete, heat to 40°C and react for 5 hours. Extract three times with 600ml of dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, concentrate under reduced pressure at 35°C to remove the solvent, and the residual liquid is 119g of 2-amino-3-methylbutyronitrile, with a yield of 87.4%.
[0031] 1.2 Preparation of 2-amino-3-methylbutanamide:
[0032] In a 500ml flask, add 6.66g of sodium hydroxide, 250ml of methanol, and 30g of distilled water, stir to dissolve the solid, add 42.5g of 2-amino-3-methylbutyronitrile, cool to below 0°C, add 29g of acetone, and React at 0-5°C for 6 hours, concentrate under reduced pressure at 38°C to recover the solven...
Embodiment 2
[0041] 2.1 Preparation of 2-amino-3-methylbutyronitrile:
[0042] In a 5000ml flask, add 1000g of 30% sodium cyanide aqueous solution, 500g of ammonium chloride, and 600g of 25% ammonia water, stir to dissolve the solid, cool to 0°C, add 500g of isobutyraldehyde dropwise, after the dropwise addition is completed, heat to 40 °C, react for 5 hours. Extract three times with 3000ml of dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, concentrate under reduced pressure at 35°C to evaporate the solvent, the residual liquid is 578g of 2-amino-3-methylbutyronitrile, the yield is 85% .
[0043]2.2 Preparation of 2-amino-3-methylbutanamide:
[0044] In a 1000ml flask, add 14 grams of sodium hydroxide, 500ml of methanol, and 50 grams of distilled water, stir to dissolve the solid, add 85g of 2-amino-3-methylbutyronitrile, cool to below 0°C, add 60 grams of acetone, and React at ~5°C for 5 hours, concentrate under reduced pressure at 38°C to recover the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com