Synthesis method of florfenicol
A technology of fluorine thiamphenicol and a synthesis method, applied in the field of chemical drugs, can solve the problems of long steps, large dosage and the like, and achieves the effects of simple operation, mild conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
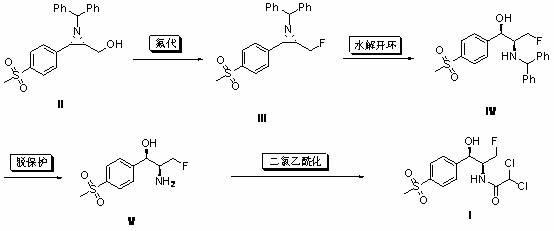
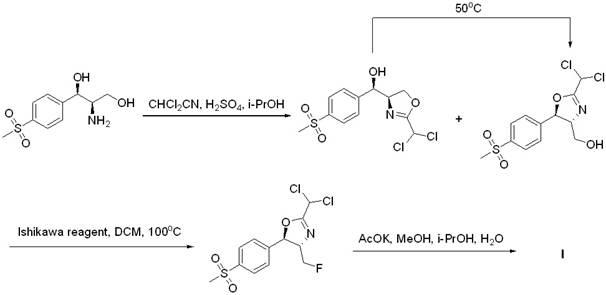
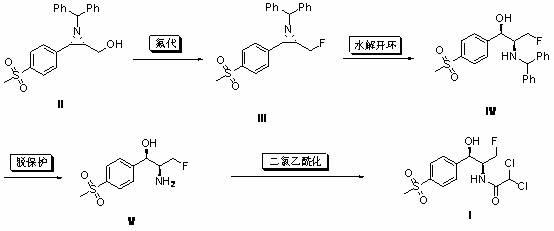
[0029] Example 11 Under the protection of nitrogen, (2S,3S)-1-benzhydryl-2-(fluoromethyl)-3-[4-(thysulfonyl)-phenyl] was sequentially added to the dry reaction flask Acridine (II) (3.93 g, 10 mmol), anhydrous dichloromethane (30 mL), DAST (3.0 g, 18 mmol), reacted at room temperature for 12 hours, quenched with 1N NaOH (30 mL), acetic acid Extracted 3 times with ethyl ester, combined, dried, filtered, concentrated, and column chromatographed to give a white solid which is compound (III) (3.80 g, 96%), mp: 166~168°C, optical rotation [α] 25 D = 101.7° (0.52, CHCl 3 ).
[0030] 1 H NMR (CDCl 3 , ppm): δ=2.40-2.47 (m, 2H, NCH), 3.03 (s, 3H, SO 2 CH 3 ), 3.10 (d, J = 6.0 Hz, 1H, ArCHN), 3.96 (s, 1H, PhCHPh), 3.98-4.12 (m, 1H, CH 2 F), 4.25-4.41 (m, 1H, CH 2 F), 7.17-7.50 (m, 10H, ArH), 7.56 (d, J = 8.4Hz, ArH), 7.86 (d, J = 8.4Hz, ArH).
[0031] ESI-MS: (m / z) = 396.1 (M + -+H).
Embodiment 12
[0032] Example 12 Under the protection of nitrogen, (2S,3S)-1-benzhydryl-2-(fluoromethyl)-3-[4-(thysulfonyl)-phenyl] was sequentially added to the dry reaction flask Acridine (II) (3.93 g, 10 mmol), anhydrous dichloromethane (30 mL), BAST (3.97 g, 18 mmol), reacted at room temperature for 12 hours, quenched with 1N NaOH (30 mL), acetic acid Ethyl ester was extracted 3 times, combined, dried, filtered, concentrated, and column chromatographed to obtain a white solid which was compound (III) (3.83 g, 97%), melting point, optical rotation, 1 H NMR and MS are consistent with Example 11.
Embodiment 13
[0033] Example 13 Under the protection of nitrogen, (2S,3S)-1-benzhydryl-2-(fluoromethyl)-3-[4-(thysulfonyl)-phenyl] was sequentially added to the dry reaction flask Acridine (II) (3.93 g, 10 mmol), anhydrous dichloromethane (30 mL), Ishikawa reagent (4.46 g, 20 mmol), sealed tube, reacted at 100°C for 6 hours, cooled to room temperature, washed with 1N NaOH ( 30 mL) to quench the reaction, extracted three times with ethyl acetate, combined, dried, filtered, concentrated, and column chromatographed to obtain a white solid which was compound (III) (2.89 g, 73%), melting point, optical rotation, 1 H NMR and MS are consistent with Example 11.
[0034] Two, (1 R ,2 S )-2-benzhydrylamino-3-fluoro-1-[4-(methylsulfonyl)-phenyl]propanol (IV) preparation
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com