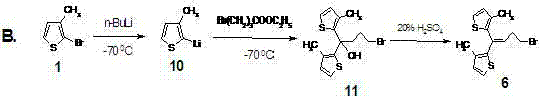Method for synthesizing tiagabine hydrochloride and method for preparing anhydrous tiagabine hydrochloride
A technology of tiagabine hydrochloride and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of easy decomposition, deepening color, low yield of alkylation, etc., and achieves the effects of mild reaction conditions and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
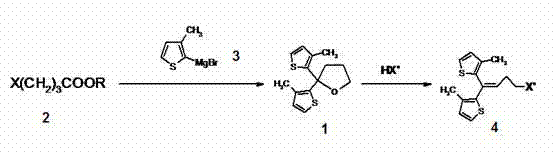
[0034] Preparation of compound 1 2,2-bis(3-methyl-2-thienyl)tetrahydrofuran
[0035] In anhydrous tetrahydrofuran (10.5 ml), magnesium chips (237 mg, 8.42×10 -3 mol) and 2-bromo3-methylthiophene (1.64 g, 9.27×10 -3 mol) to prepare (3-methyl-2-thienyl)magnesium bromide in THF, cooled to 0 °C with ice water, added ethyl 4-bromobutyrate (0.897 g, 4.60 ×10 -3 mol), control the addition below 10 °C, and then heat up and reflux for 3 h. After the reaction mixture was cooled to 0°C, saturated NH 4 Cl solution (20.0 ml). The organic phase was separated, and the aqueous phase was extracted with ethyl acetate (6 ml×2). Combine the organic phases and dilute with anhydrous Na 2 SO 4 Drying. The organic phase was rotary evaporated to obtain the compound 1 : Pale yellow solid, dissolved in a small amount of ethanol, added petroleum ether to precipitate the compound; white crystal (1.11g, yield 91.1%); Mp: 68-72 °C; MS ESI + 265 [M+1] + ; 1 H NMR (CDCl 3): d 2.10 (s, 3H), 2.12...
Embodiment 2
[0037] Compound 4 4-Bromo-1.1-bis(3-methyl-2-thienyl)-1-butene
[0038] compound 1 (1.21 g, 4.58 ×10 -3 mol) with 47.0% HBr (7.26 g, 42.1×10 -3 mol) mixture was stirred and heated at 80 °C for 6 h. After cooling to room temperature, extract with ethyl acetate 10ml ?? 2, combine the organic phases, and successively add water, 1% K 2 CO 3 Aqueous solution and washed to neutral. organic phase with anhydrous Na 2 SO 4 After drying and decolorization with activated carbon, the purple oil was obtained by rotary evaporation, and then purified by column chromatography (silica gel 300-400 mesh, petroleum ether) to obtain the compound 4 : yellow oil (1.31 g, yield 87.5%); MS ESI + : 327 [M+1] + ; IR (KBr): = 3103, 2962, 1443, 1267, 712 cm -1 ; MS ESI + : 328 [M+1] + ; 1 H NMR (CDCl 3 ) d 2.05 (s, 3H), 2.07 (s, 3H), 2.72 (q, J = 7.2 Hz, 2H), 3.45 (t, J = 7.0 Hz, 2H), 6.08 (t, J = 7.2 Hz, 1H), 6.80 (d, J = 5.2 Hz, 1H), 6.87 (d, J = 5.2 Hz, 1H), 7.08 (d, J...
Embodiment 3
[0040] Compound 6 ( R )-(-)-N-[4,4-bis(3-methylthiophen-2-yl)-3-butenyl]-3-piperidinecarboxylic acid ethyl ester [tiagabine ethyl ester]
[0041] at room temperature R -Ethyl 3-piperidinecarboxylate (2.27 g, 14.4 × 10 -3 mol), 7 (4.90 g, 14.4 × 10 -3 mol), anhydrous K 2 CO 3 (2.98 g, 21.6×10 -3 mol), KI (120 mg, 7.2×10 -4 mol) and acetone (30 ml) were stirred for 72 hours, filtered to remove inorganic salts, the filtrate was rotary evaporated, and the residue was purified by column chromatography (silica gel 200-300 mesh, petroleum ether: acetone) to obtain the compound 6 : yellow oil (4.28 g, yield 74.3%); [a] D 25 = -24.9° ( c = 1.00, EtOH); IR (KBr): =3104, 2942, 1732, 1446, 712cm -1 ; MS (ESI + ): 404.2 [M+1] + ; 1 H-NMR (500MHz, CDCl 3 ) d: 1.24 (t, J = 7.1 Hz, 3H), 1.41 (ddd, J = 3.5 / 12.7 / 16.2 Hz, 1H), 1.50~1.60 (m, 1H), 1.62~1.75 (m, 1H), 1.87~1.99 (m, 2H), 2.00 (s, 3H), 2.04 (s, 3H ), 2.10~2.17 (m, 1H), 2.26 (q, J = 5.5 Hz, 2H), 2.45~2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com