Transdermal patch containing fentanyl or salt thereof
An absorption type and patch technology, applied in the field of percutaneous absorption type patch, can solve the problems of cancer patients unable to stably administer drugs, unknown stabilization effect, and reduced patient compliance, and achieve excellent adhesion and penetration. Excellent skin properties, excellent stability over time, and excellent drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
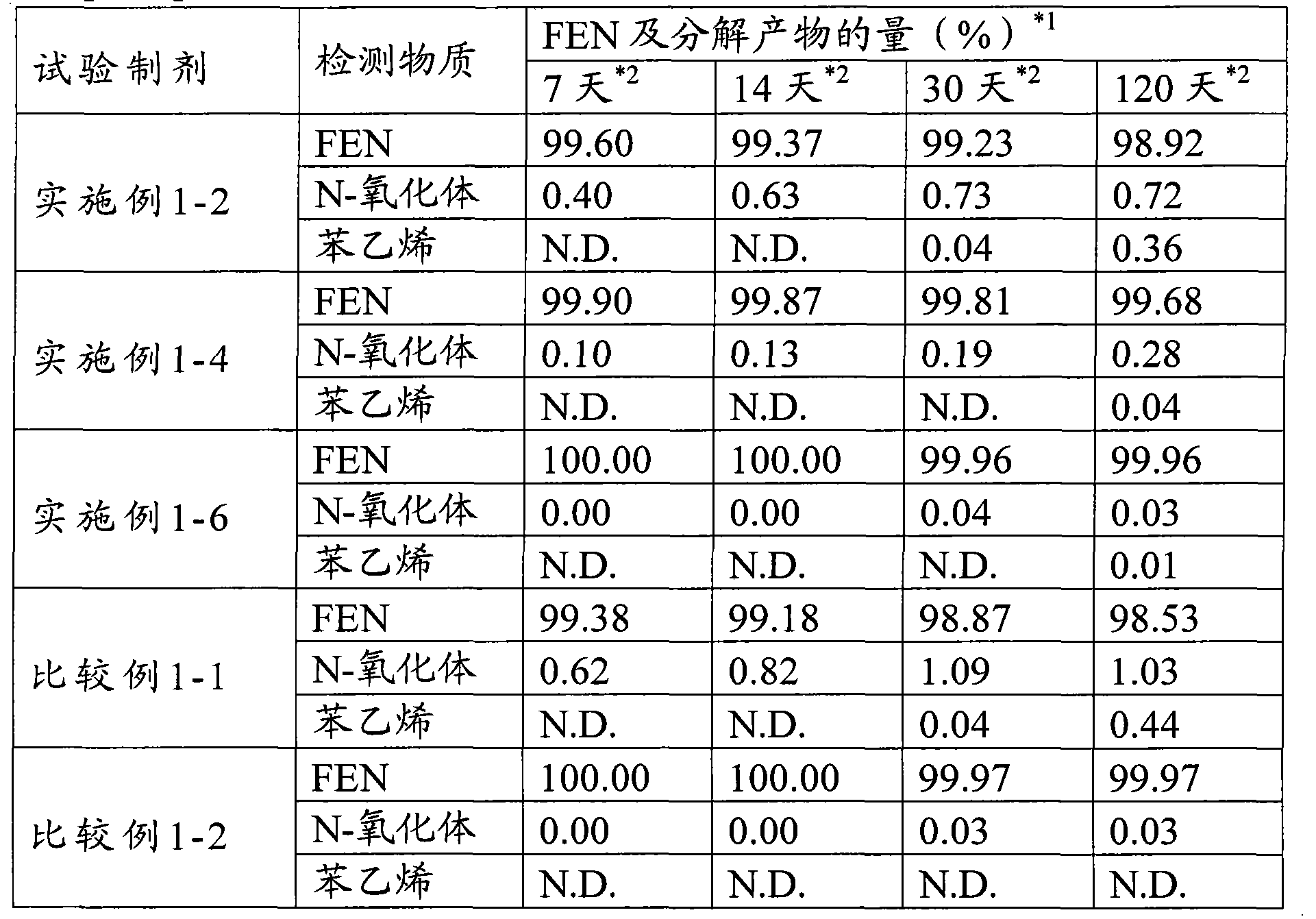
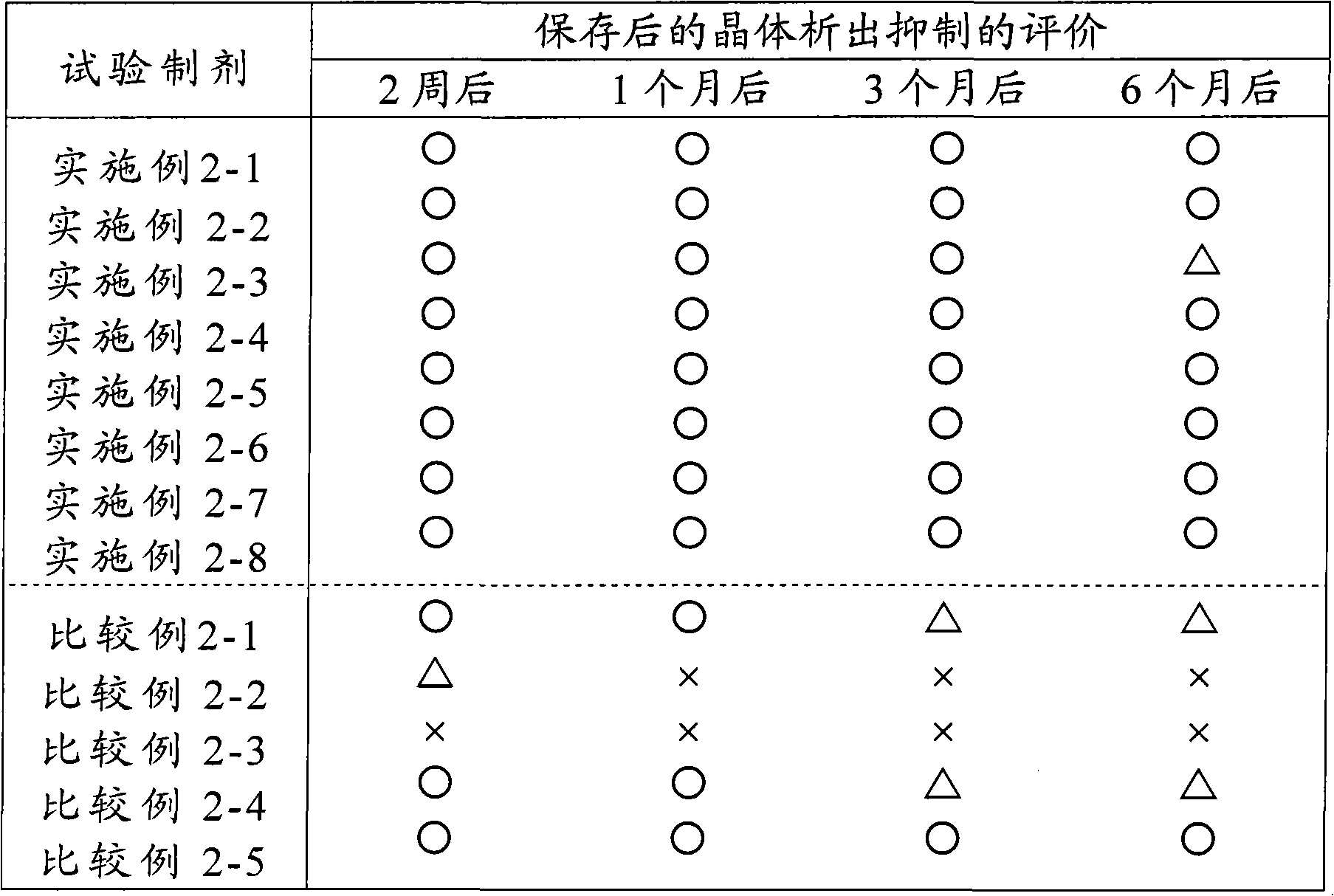
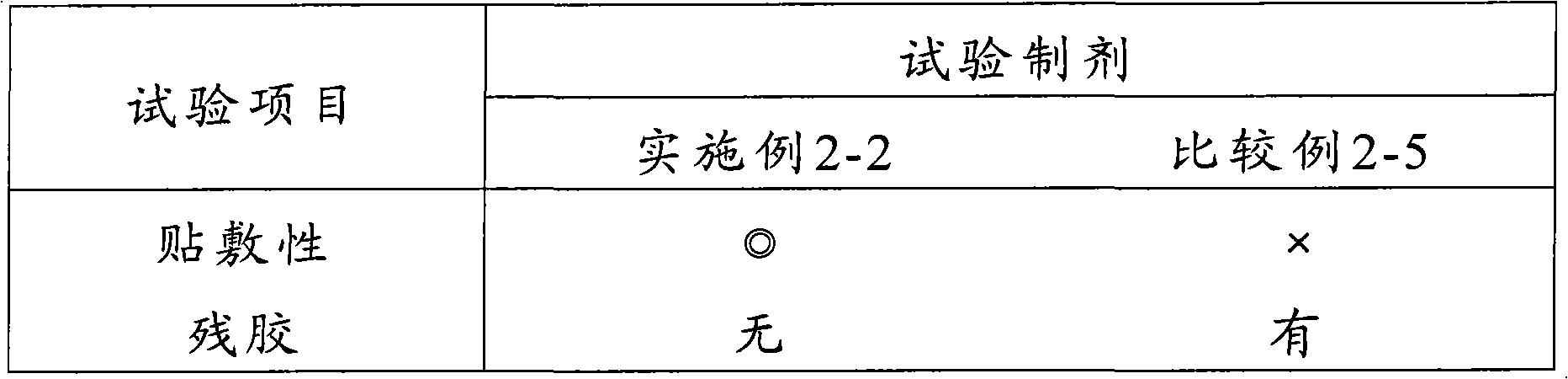
Examples
Embodiment 1-1
[0072] Embodiment 1-1 Fentanyl-containing patch (1-1)
[0073] A transdermal patch of the following formulation was prepared according to the following production method.
[0074] (formula)
[0075] Fentanyl 1.0%
[0076] Styrene-isoprene-styrene block copolymer * 18.2%
[0077] Polyisoprene 9.8%
[0078] Hydrogenated Rosin Glycerides ** 1.0%
[0079] polyterpene resin *** 41.99%
[0080] Polybutene 14.0%
[0081] Liquid paraffin 14.0%
[0082] L-Ascorbyl Palmitate 0.01%
[0083] Total 100%
[0084] * : JSR Kraton elastomer, Kraton D-KX401CS
[0085] ** : Arakawa Chemical Industry, Pine Crystal KE-311
[0086] *** : Arakawa Chemical Industry, Alcon P-100
[0087] (preparation method)
[0088] Add styrene-isoprene-styrene block copolymer, polyisoprene, hydrogenated rosin glyceride, polyterpene resin, polybutene, liquid paraffin to toluene, and L - Ascorbyl palmitate, stir to mix, then add fentanyl, stir to mix to get...
Embodiment 1-2
[0089] Embodiment 1-2 Fentanyl-containing patch (1-2)
[0090] A transdermal patch of the following formulation was prepared according to the following production method.
[0091] (formula)
[0092] Fentanyl 4.0%
[0093] Styrene-isoprene-styrene block copolymer * 18.2%
[0094] Polyisoprene 9.8%
[0095] Hydrogenated Rosin Glycerides ** 5.0%
[0096] polyterpene resin *** 34.98%
[0097] Polybutene 14.0%
[0098] Liquid paraffin 14.0%
[0099] L-Ascorbyl Palmitate 0.02%
[0100] Total 100%
[0101] * , ** and *** : Same as Example 1-1.
[0102] (preparation method)
[0103] Add styrene-isoprene-styrene block copolymer, polyisoprene, hydrogenated rosin glyceride, polyterpene resin, polybutene, liquid paraffin to toluene, and L - Ascorbyl palmitate, stir to mix, then add fentanyl, stir to mix further to get a homogeneous lysate. Then use the knife coater to apply the dissolved product on the peeling film, then ai...
Embodiment 1-3
[0104] Embodiment 1-3 contains fentanyl type patch (1-3)
[0105] A transdermal patch of the following formulation was prepared according to the following production method.
[0106] (formula)
[0107] Fentanyl 6.0%
[0108] Styrene-isoprene-styrene block copolymer * 18.2%
[0109] Polyisoprene 9.8%
[0110] Hydrogenated Rosin Glycerides ** 10.0%
[0111] polyterpene resin *** 27.5%
[0112] Polybutene 14.0%
[0113] Liquid paraffin 14.0%
[0114] L-Ascorbyl Palmitate 0.5%
[0115] Total 100%
[0116] * , ** and *** : Same as Example 1-1.
[0117] (preparation method)
[0118] Add styrene-isoprene-styrene block copolymer, polyisoprene, hydrogenated rosin glyceride, polyterpene resin, polybutene, liquid paraffin to toluene, and L - Ascorbyl palmitate, stir to mix, then add fentanyl, stir to mix further to get a homogeneous lysate. Next, apply this solution on a release film using a knife coater, then air-dry it, and af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com