Lithium iron phosphate composite anode material in lithium-ion battery and preparation method thereof
A composite positive electrode material and lithium-ion battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as difficult practical application, low lithium ion diffusion rate, and low electronic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Mix analytically pure lithium carbonate, ferrous oxalate, and ammonium dihydrogen phosphate according to the stoichiometric ratio of 0.5:1:1, then add a small amount of glucose, use absolute ethanol as the medium, wet ball mill for 20 hours, and dry to obtain the precursor. The precursor was placed in a tube furnace, protected by argon, heated to 350°C for 5 hours, then heated to 700°C for 12 hours and then cooled to room temperature with the furnace. Add nickel nanowires with a mass fraction of 0.5wt% to the above product, use ethanol as a dispersant, stir and sonicate to make it evenly mixed, after drying, keep it in a vacuum oven at 200°C for 1 hour to obtain the final product.
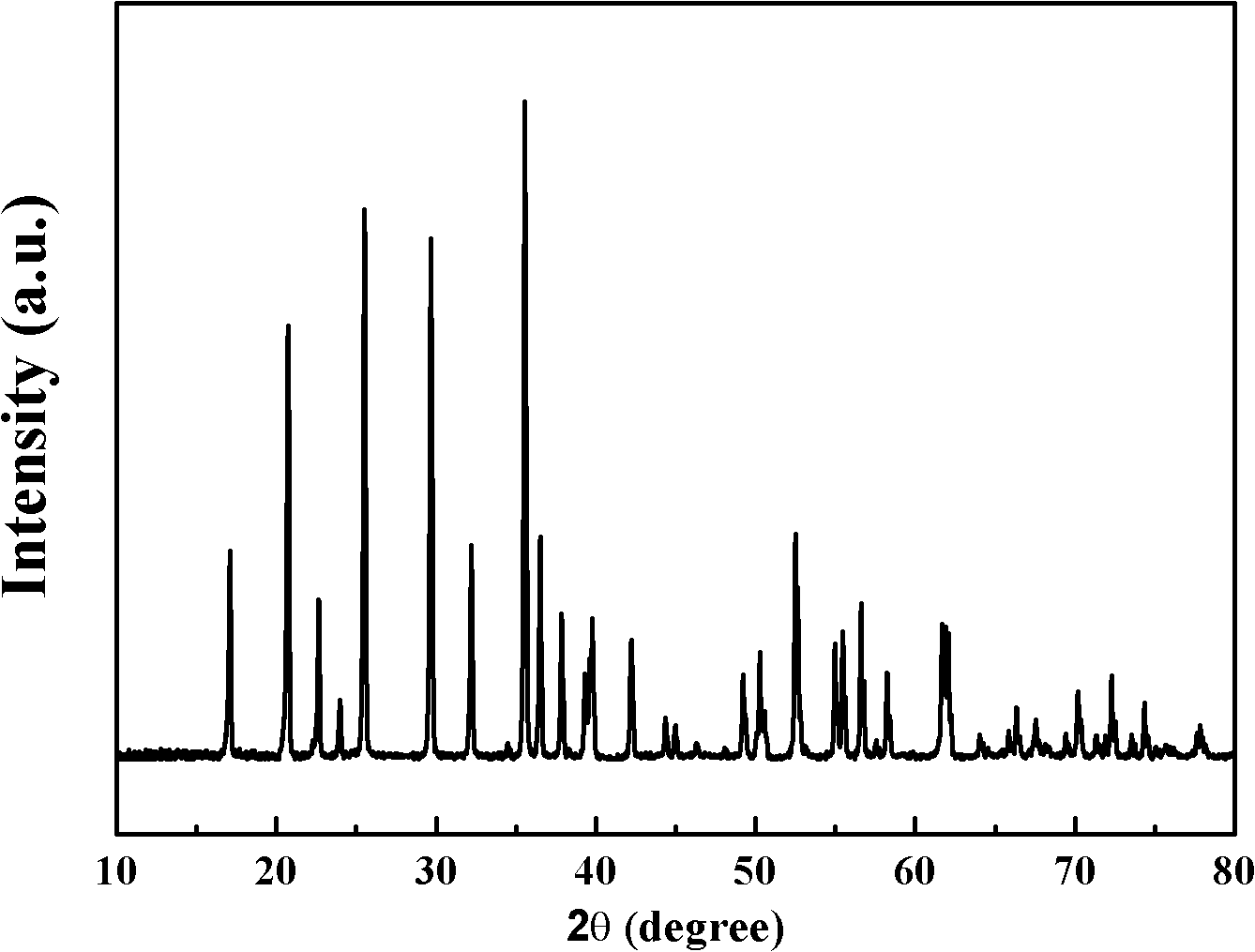
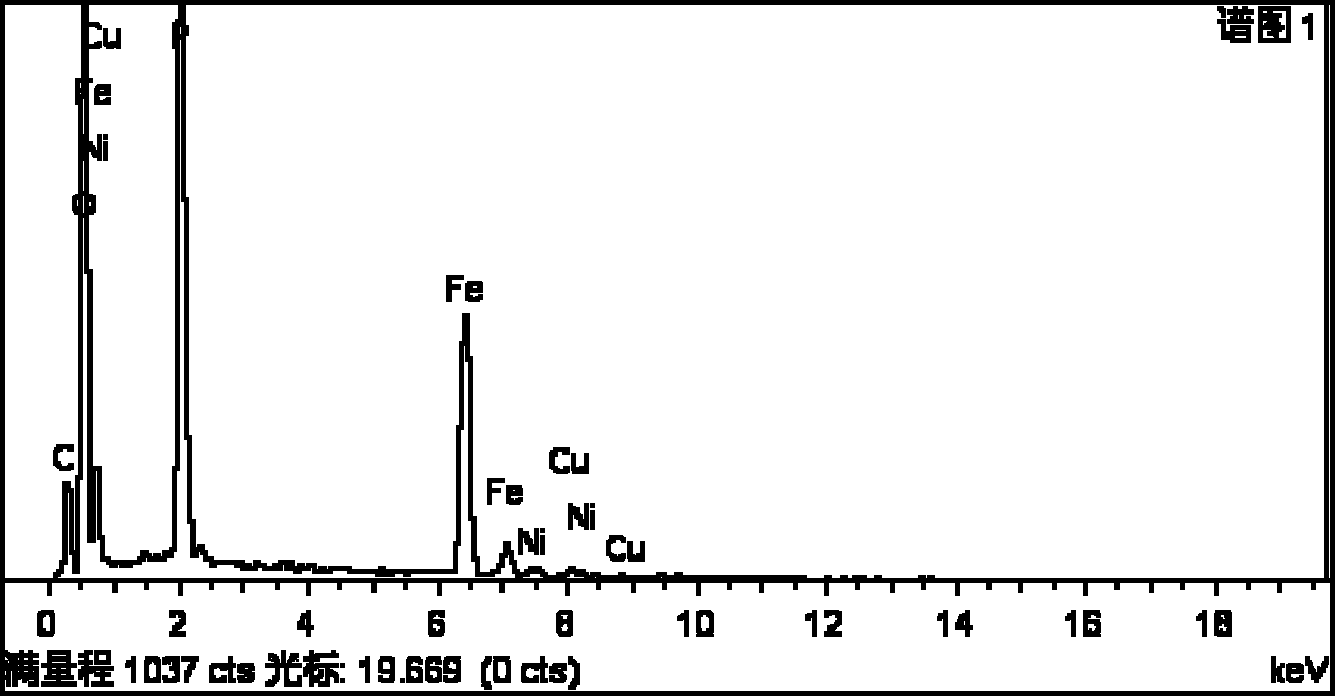
[0028] The XRD pattern of the prepared lithium-ion battery cathode material is as follows figure 1 As shown, the SEM image is shown in figure 2 As shown, the EDS diagram is as image 3 shown. It can be seen that the obtained product is olivine-type lithium iron phosphate with a complete ...
Embodiment 2
[0033]Mix analytically pure lithium carbonate, ferrous oxalate, and ammonium dihydrogen phosphate according to the stoichiometric ratio of 0.5:1:1, then add a small amount of sucrose, use absolute ethanol as the medium, wet ball mill for 24 hours, and dry to obtain the precursor. The precursor was placed in a tube furnace, protected by argon, heated to 350°C for 10 hours, then heated to 700°C for 20 hours, and then cooled to room temperature with the furnace. Add aluminum oxide nanowires with a mass fraction of 1 wt% to the above product, then add ethanol as a dispersant, stir vigorously to make it evenly mixed, and after drying, keep warm at 220°C for 1 h in an argon atmosphere to obtain the final product.
Embodiment 3
[0035] Mix analytically pure lithium hydroxide, ferrous oxalate, and ammonium dihydrogen phosphate according to the stoichiometric ratio of 1.05:1:1, then add a small amount of glucose, use absolute ethanol as the medium, wet ball mill for 18 hours, and dry to obtain the precursor. The precursor was placed in a tube furnace, protected by nitrogen, heated to 300°C for 6 hours, then heated to 750°C for 15 hours, and then cooled to room temperature with the furnace. Add nickel oxide nanowires with a mass fraction of 0.3wt% to the above product, then add ethanol as a dispersant, stir vigorously to make it evenly mixed, and after drying, keep warm at 300°C for 30 minutes in a nitrogen atmosphere to obtain the final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com