Preparation method of electrolyte for vanadium redox battery (VRB)
A technology for all-vanadium redox flow batteries and electrolytes, which is applied in the field of preparation of electrolytes for all-vanadium redox flow batteries, can solve the problems of low solubility, small preparation volume, and difficult purification, and achieve the effect of simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
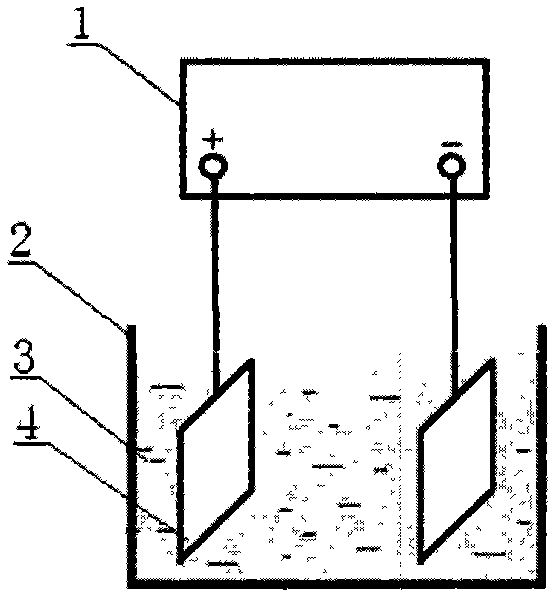
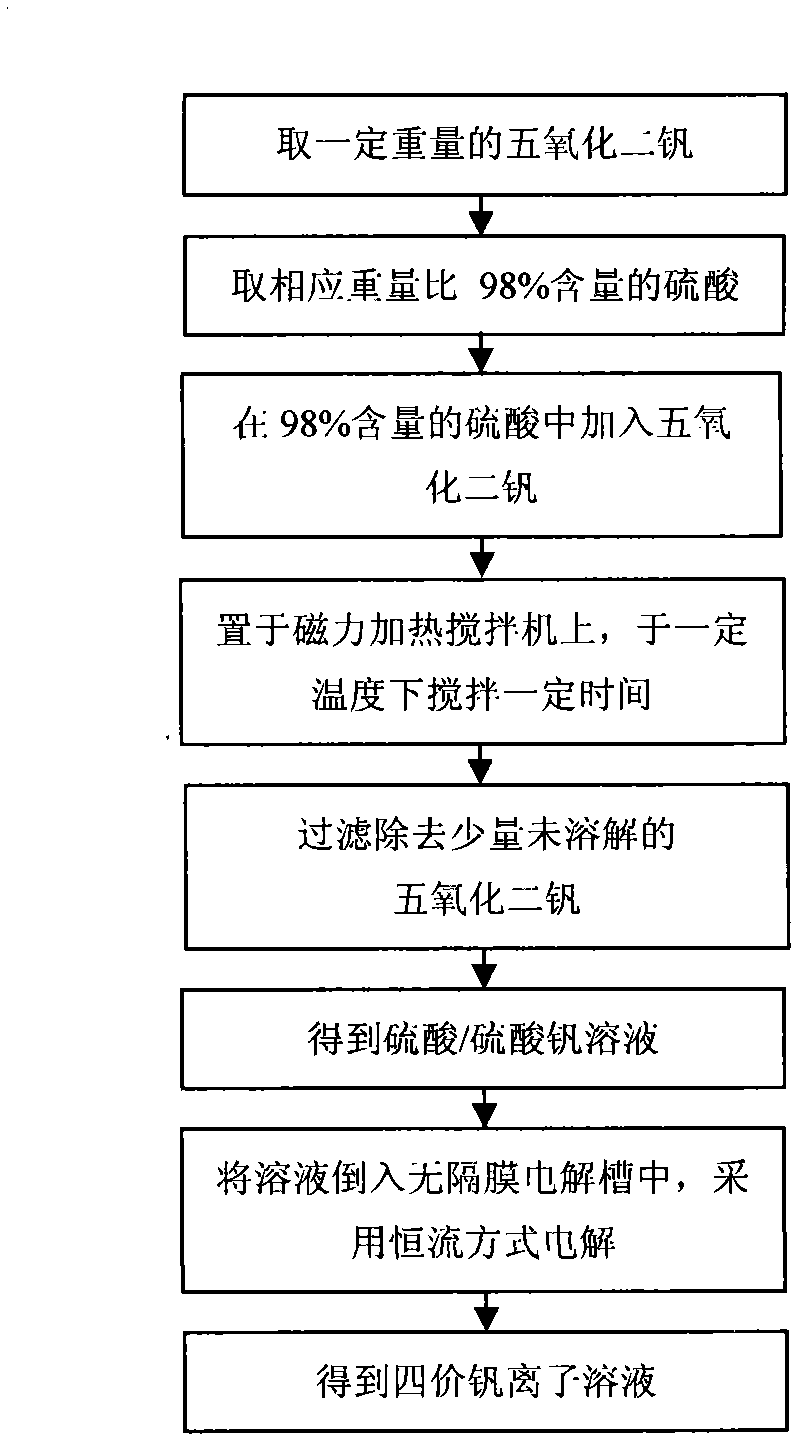
[0033] Take by weighing 50g of vanadium pentoxide, 125g of 98% sulfuric acid and appropriate amount of distilled water. Add 50g of vanadium pentoxide powder to 165.6g of 98% sulfuric acid, place it on a magnetic heating stirrer, stir at 140°C for 0.5h, filter out a small amount of insoluble vanadium pentoxide, and obtain sulfuric acid and vanadium sulfate the mixed solution 3; get 50ml of this mixed solution and add it into the electrolytic cell 2 without diaphragm, use the direct current constant voltage constant current power supply 1, put the lead plate electrode 4 that is connected with the direct current constant voltage constant current power supply 1 into the place filled with sulfuric acid and In the electrolytic cell 2 without diaphragm of the vanadium sulfate mixed solution 3, a constant current electrolysis method is adopted, and the current density is 300mA / cm 2 After 20 hours of electrolysis, a solution of tetravalent vanadium ions was obtained; the electrolytic s...
Embodiment 2
[0035] Weigh 50g of vanadium pentoxide, 200g of 98% sulfuric acid and appropriate amount of distilled water according to the proportioning ratio. Add vanadium pentoxide powder to 165.6g of 98% sulfuric acid, place it on a magnetic heating stirrer, stir at 120°C for 1 hour, filter out a small amount of insoluble vanadium pentoxide, and obtain a mixture of sulfuric acid and vanadium sulfate Solution 3: Take 50ml of the mixed solution and add it to the electrolytic cell 2 without a diaphragm, use a DC constant voltage and constant current power supply 1, and put the lead plate electrode 4 connected with the DC constant voltage and constant current power supply 1 into a tank containing sulfuric acid and vanadium sulfate In the electrolytic cell 2 without diaphragm of the mixed solution 3, a constant current electrolysis method is adopted, and the current density is 200mA / cm 2, electrolyzed for 19 hours to obtain a solution of tetravalent vanadium ions; the electrolytic solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com