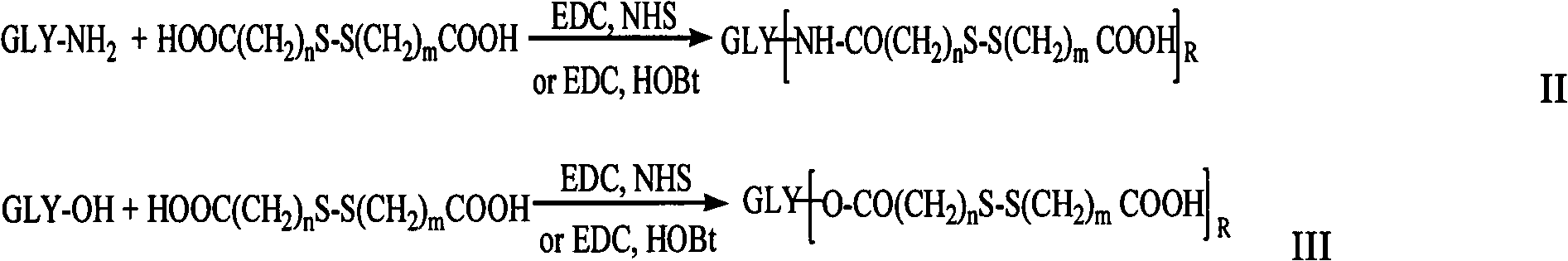Amphiphilic polysaccharide derivative vector for specific medicine release in organism focusas well as preparation and application of pharmaceutical composition thereof
An amphiphilic polysaccharide, specific technology, applied to the preparation of the carrier, amphiphilic polysaccharide derivatives as drug carriers, can solve the problems of slow hydrophobic segment shedding, unfavorable curative effect, etc., to achieve regular particles without adhesion, re- Good dispersion and the effect of increasing the concentration of free drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: Preparation of octanoylcystamine hyaluronic acid
[0078] 0.1mmol of hyaluronic acid, 1mmol of cystamine, 0.2mmol of EDC and 0.2mmol of NHS were dissolved in formamide. After 24 hours of reaction, the intermediate of hyaluronic acid was precipitated with acetone, filtered with suction and dialyzed with distilled water for 3 days (MWCO=3500) to obtain the free end Amino hyaluronic acid intermediate.
[0079] Dissolve 0.4mmol octanoic acid and 0.1mmol intermediate in formamide, 0.4mmol EDC is used as the activator, and react for 24h. After the reaction, use excess acetone to precipitate, filter and vacuum dry to obtain the hyaluronic acid derivative carrier modified by octanylamino group.
Embodiment 2
[0080] Embodiment 2: the preparation of lauryl-3,3'-dithiodipropionic acid chitosan
[0081] 0.1 mmol of chitosan was dissolved in a mixed solvent of water and methanol (v / v=1:1), 1 mmol of 3,3'-dithiodipropionic acid, 0.1 mmol of EDC and 0.1 mmol of HOBt were added, reacted for 8 h, and methanol was removed by rotary evaporation. Distilled water was dialyzed for 3 days (MWCO=3500) to obtain a chitosan intermediate with a free carboxyl group at one end.
[0082] 0.2 mmol dodecylamine and 0.1 mmol chitosan intermediate were dissolved in a mixed solvent of water and methanol (v / v=1:1), 0.2 mmol EDC was used as an activator, and the reaction was carried out for 24 hours. Methanol was removed by rotary evaporation, dialyzed in distilled water for 3 days (MWCO=3500), and freeze-dried to obtain the chitosan derivative carrier modified by dodecylamide group.
Embodiment 3
[0083] Embodiment 3: Preparation of deoxycholic acid-cystamine chondroitin sulfate
[0084] 0.1mmol of chondroitin sulfate, 2mmol of cystamine, 0.4mmol of EDC and 0.4mmol of NHS were dissolved in formamide. After 12 hours of reaction, the intermediate of chondroitin sulfate was precipitated with acetone, filtered with suction and dialyzed with distilled water for 3 days (MWCO=3500) to obtain the free end Amino chondroitin sulfate intermediate.
[0085] 0.5mmol deoxycholic acid, 0.65mmol dicyclohexylcarbodiimide (DCC), and 0.65mmol hydroxysuccinimide (NHS) were dissolved in N, N'-dimethylformamide, reacted in ice bath for 30min, and then raised Reaction at room temperature for 24h. After the reaction, the precipitate was filtered off, precipitated with excess tetrahydrofuran, and filtered to obtain N-hydroxysuccinimide (NHS) active ester of deoxycholic acid.
[0086] 0.4 mmol of deoxycholic acid N-hydroxysuccinimide (NHS) active ester and 0.1 mmol of chondroitin sulfate inter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com