Method for disproportionating methyl chlorosilane
A technology of methyl chlorosilane and methyl trichlorosilane, applied in the field of methyl chlorosilane disproportionation, can solve the problems of high reaction temperature and pressure, difficult separation, increased trimethyl output, etc., and achieves easy control of conditions and high conversion rate of raw materials , good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
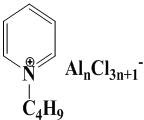
[0030] Preparation of Chloroaluminate Ionic Liquid Catalyst
[0031] N -Butylpyridinechloroaluminate[BPy]Cl-nAlCl 3(n=2-6) Preparation method of ionic liquid: under nitrogen protection, 1.50 mol (119 g) of pyridine, 1.7 mol (158 g) of chlorobutane Stir and mix well, then warm up to 100 °C in the dark to appear a gentle reflux, and react at this temperature for 72 h. Stop heating, cool to below 10 °C, white crystals precipitated, filter with suction and wash the crude product with ethyl acetate, and dry the product in vacuum at 70 °C for 48 h to remove residual ethyl acetate. The yield of the product [BPy]Cl is about 55%, and it is stored in a dry and inert atmosphere for future use.
[0032] The glove box was filled with nitrogen, and different amounts of anhydrous AlCl were slowly added in batches to the quantitative above-mentioned intermediate [BPy]Cl at room temperature. 3 During the feeding process, keep stirring to avoid local high temperature until the solids are ...
Embodiment 1
[0034] Methyltrichlorosilane and low boiler disproportionation reaction to prepare dimethyldichlorosilane:
[0035] Add methyl trichlorosilane and low boiler mass ratio 1.5:1 total 150 g reaction mixture and 16.7% (25 g) aluminum trichloride and chloride in autoclave N -Butylpyridine molar ratio 6:1 N -Butylpyridine chloroaluminate [BPy]Cl-6AlCl 3 The ionic liquid catalyst was sealed, and the air in the kettle was replaced by nitrogen gas, kept fully stirred (500 rpm), heated to 150 °C, and reacted for 5 h. After the reaction is finished, the temperature of the reaction kettle is lowered to room temperature, and the reaction product is taken out, and the product is separated from the ionic liquid, and then the product is sampled and analyzed by gas chromatography immediately.
[0036] The gas chromatography analysis conditions are:
[0037] Chromatographic column: OV-1701 capillary column; detector type; FID; carrier gas: high-purity nitrogen; injector temperature: 180 ℃;...
Embodiment 2
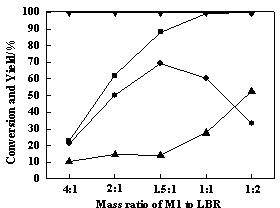
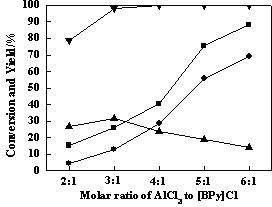
[0046] Same as Example 1 but keep methyltrichlorosilane and low boiler mass ratio 1.5:1, only change aluminum trichloride and chloride in ionic liquid N -Butylpyridine molar ratios are 2:1, 3:1, 4:1, 5:1 respectively, the results are shown in image 3 .
[0047] Depend on image 3 It can be seen that with [BPy]Cl-nAlCl 3 AlCl in ionic liquid catalyst 3 With the continuous increase of the molar ratio of [BPy]Cl, the conversion rate of tetramethylsilane in low boilers increased rapidly to 100%, and the conversion rate of methyltrichlorosilane and the yield of dimethyldichlorosilane showed an obvious increasing trend . When AlCl 3 When the molar ratio with [BPy]Cl was 6:1, the conversion rate of methyltrichlorosilane reached 88.21%, the yield of dimethyldichlorosilane reached 69.40%; the yield of trimethylchlorosilane was Continuous decline. Therefore, increasing [BPy]Cl-nAlCl 3 AlCl in ionic liquid catalyst 3 The content of has a significant impact on the disproportiona...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com