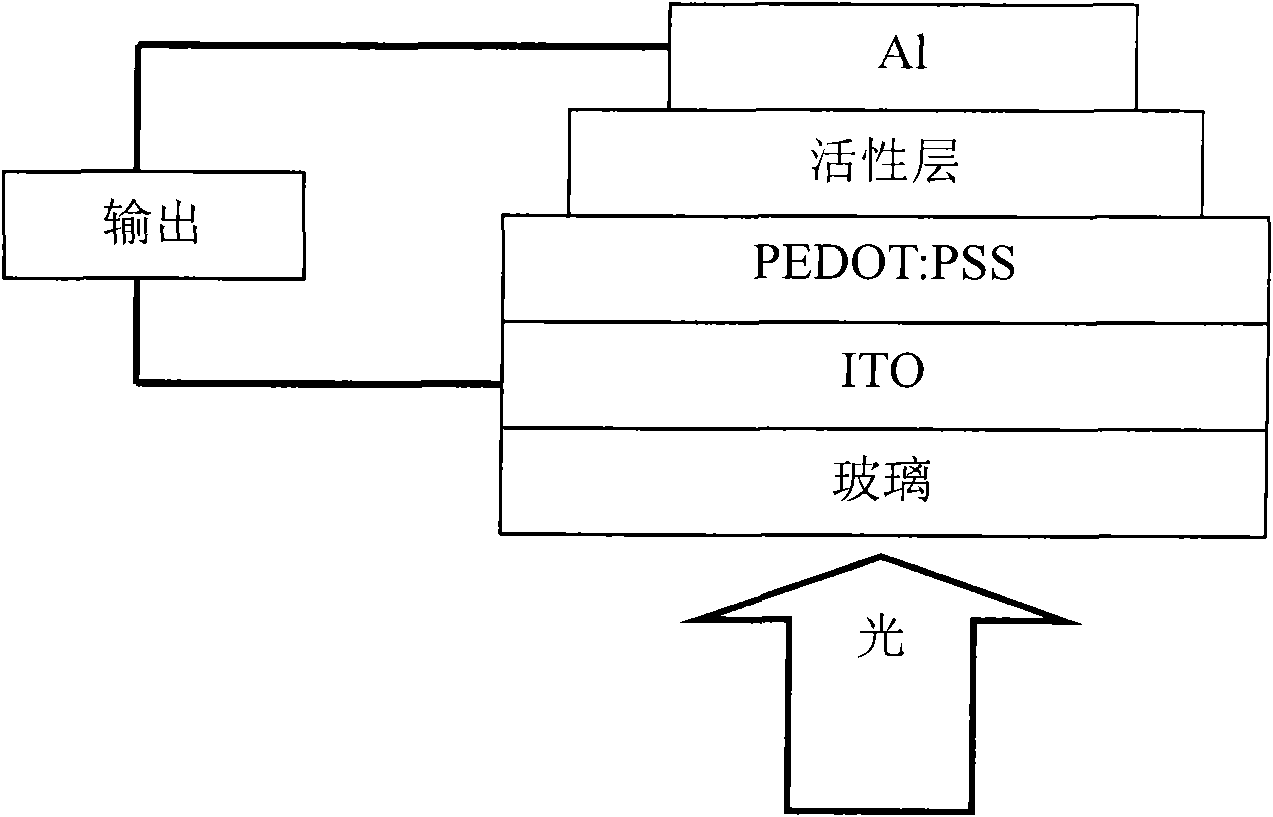
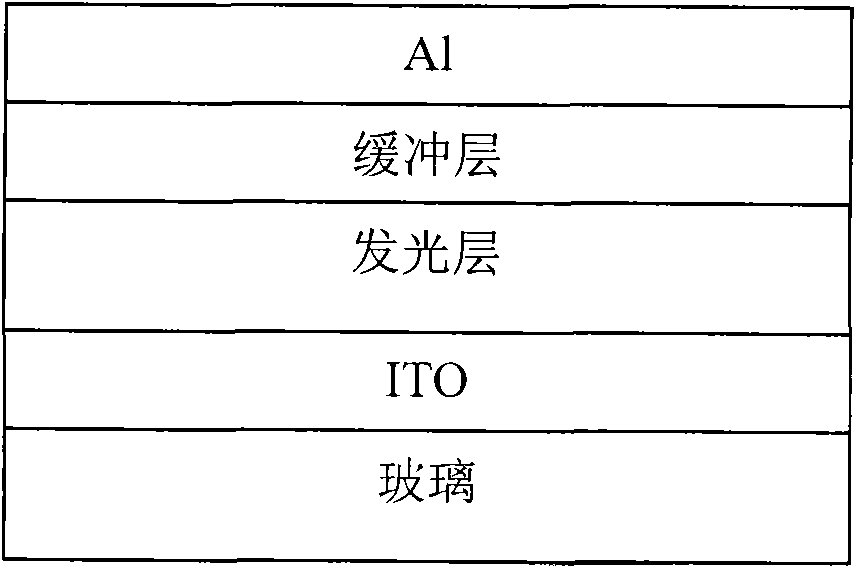
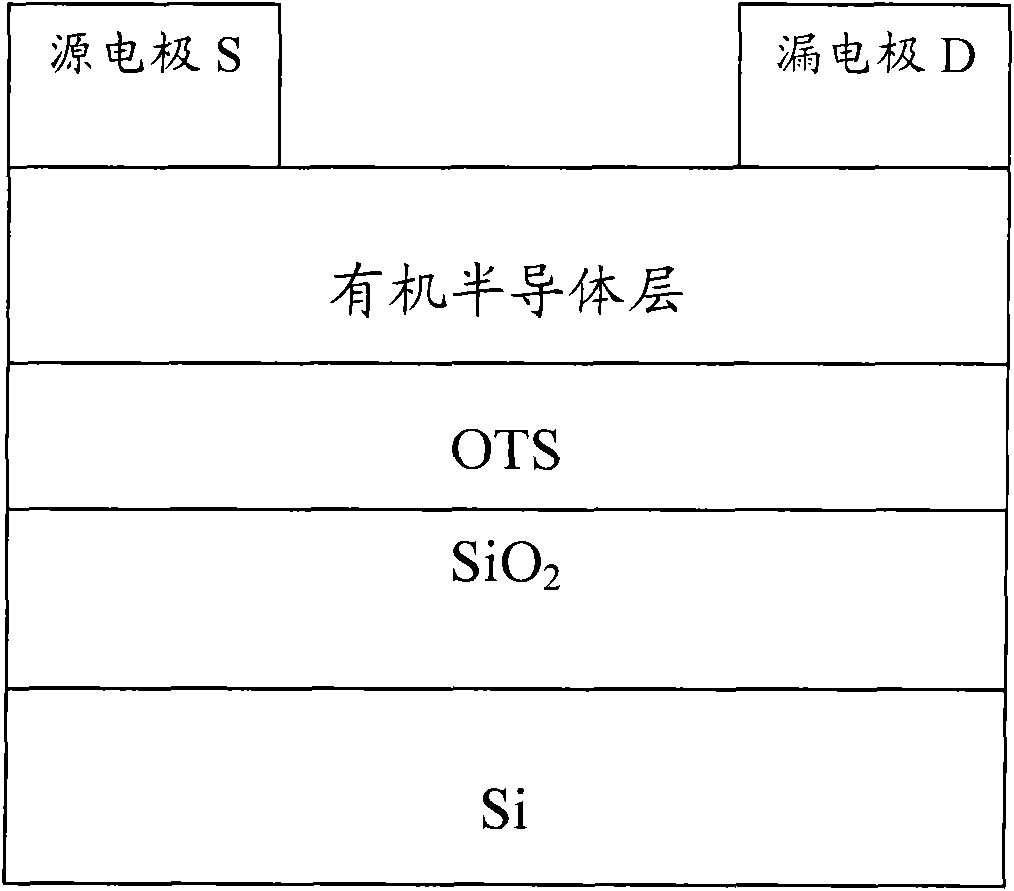
Perylene tetracarboxylic dianiline conjugated polymer and preparation method and application thereof
A perylenetetracarboxylic acid diimide and conjugated polymer technology, which is applied in the field of conjugated polymers and its preparation, can solve the problems of poor film-forming processability, poor solar light efficiency, and poor solubility, and achieve enhanced Effects of flatness and conjugation, wide absorption range, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Regarding poly-N,N'-bis-(3,4,5-tri-dodecyloxybenzene)-3,4,9,10-perylenediimide-4,7-bis(3,6- The preparation of dioctylthieno[3,2-b]thiophene)-2,1,3-benzothiadiazole, the chemical reaction formula is as follows:
[0068]
[0069] Under the protection of nitrogen, the compound N,N'-bis-(3,4,5-tri-dodecyloxybenzene)-1,7-dibromo-3,4,9,10-perylenediyl Imine 0.5mmol, 4,7-bis(5-tributyltin-(3,6-dioctylthieno[3,2-b]thiophene))-2,1,3-benzothiadiazole 0.5mmol Toluene (20mL) solution, bubble 0.5h to remove residual oxygen, then add Pd 2 (dba) 3 (0.014g, 0.015mmol) and P(o-Tol) 3 (0.0093g, 0.030mmol) and bubbling for 0.5h to remove residual oxygen, then heated to 80 ° C for 48 hours; the mixture was added dropwise to methanol for sedimentation, filtered, washed with methanol, dried, and then dissolved with toluene, Add it to the aqueous solution of sodium diethyldithiocarbamate, then heat the mixture to 90°C and stir overnight; pass the organic phase through alumina column c...
Embodiment 2
[0071] About poly-N,N'-bis-(3,4,5-tri-octyloxybenzene)-3,4,9,10-perylenediimide-4,7-bis(3,6-dioctyl The preparation of thieno[3,2-b]thiophene)-2,1,3-benzothiadiazole, the chemical reaction formula is as follows:
[0072]
[0073] Under the protection of nitrogen, to the compound N,N'-bis-(3,4,5-tri-octyloxybenzene)-1,7-dibromo-3,4,9,10-perylenediimide 0.5mmol, 4,7-bis(5-tributyltin-thieno[3,2-b]thiophene))-2,1,3-benzothiadiazole 0.5mmol in toluene / THF (30mL) solution, drum Soak for 0.5h to remove residual oxygen, then add Pd(PPh 3 ) 4 0.0069mmol, bubbled for 0.5h to remove residual oxygen, and then heated to 80°C for 72 hours. Add the mixed solution dropwise to methanol for sedimentation, filter with suction, wash with methanol, dry, then dissolve with toluene, add it to the aqueous solution of sodium diethyldithiocarbamate, then heat the mixed solution to 80°C and stir overnight. The organic phase was subjected to column chromatography of alumina, rinsed with chloroben...
Embodiment 3
[0075] About poly N,N'-bis-(3,5-di-eicosyloxy-4-toluene)-3,4,9,10-perylenediimide-4,7-bis(6-deca The preparation of dialkoxythieno[3,2-b]thiophene)-2,1,3-benzothiadiazole, the chemical reaction formula is as follows:
[0076]
[0077] Under the protection of nitrogen, the compound N,N'-bis-(3,5-di-eicosyloxy-4-toluene)-1,7-dibromo-3,4,9,10-perylene bis Imide 0.52mmol, 4,7-bis(5-tributyltin-(6-dodecyloxythieno[3,2-b]thiophene))-2,1,3-benzothiadiazole 0.5 mmol of toluene (20mL) solution, bubbled for 0.5h to remove residual oxygen, then added Pd(PPh 3 ) 2 Cl 2 0.0071mmol, bubbling for 0.5h to remove residual oxygen, then heated to 100°C for 56 hours, the mixture was added dropwise to methanol for sedimentation, suction filtered, washed with methanol, dried, then dissolved in toluene, added to diethyl In an aqueous solution of sodium dithiocarbamate, then heat the mixture to 80°C and stir overnight; pass the organic phase through alumina column chromatography, rinse with c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com