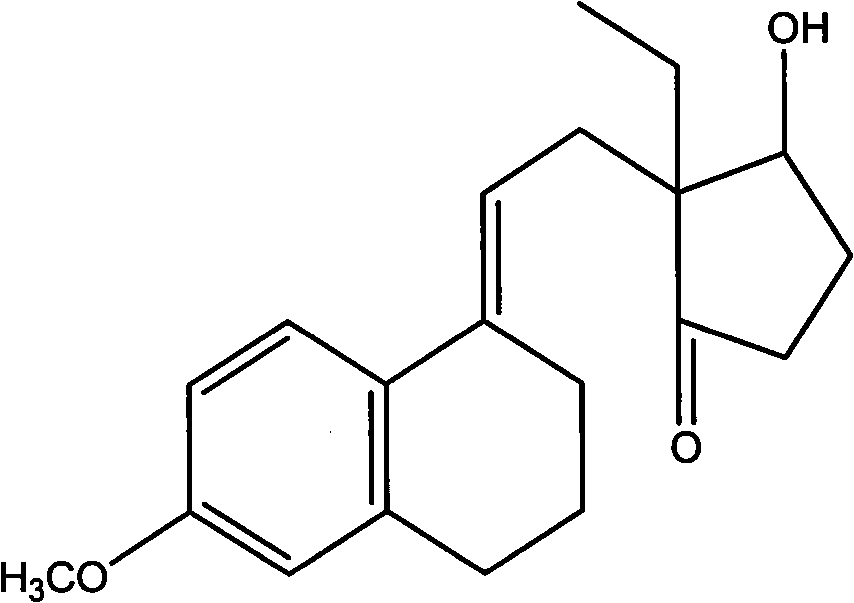
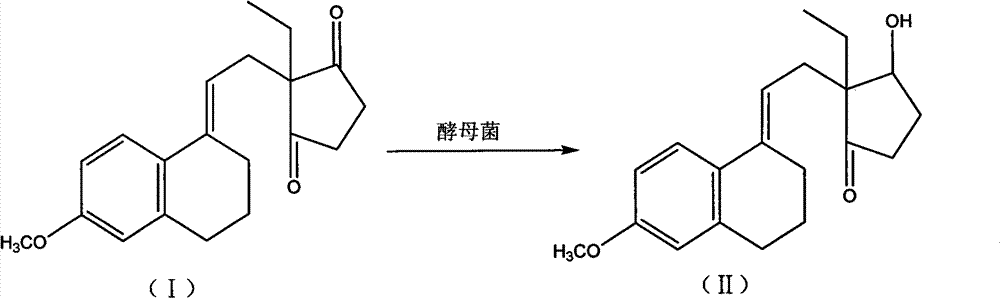
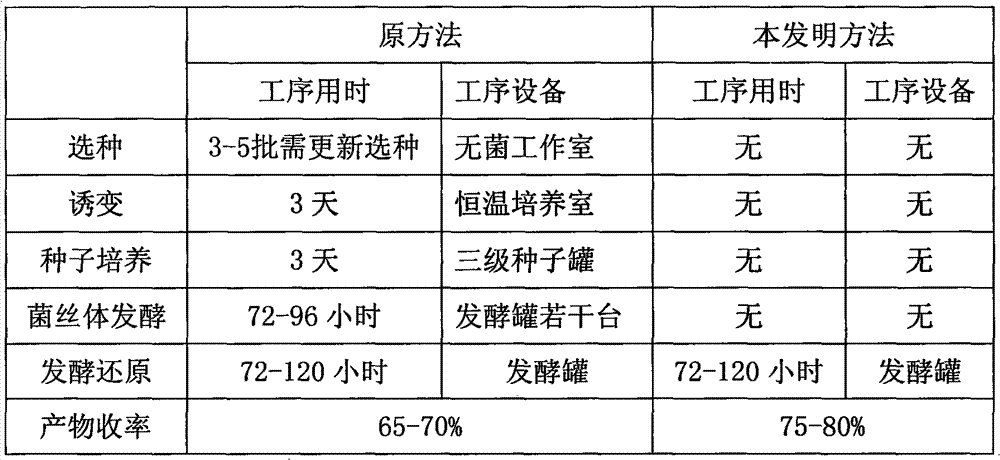
Novel fermentation reducing method for contraceptive intermediate
An intermediate and new method technology, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of low reduction efficiency, production cycle, material consumption and energy consumption, unsatisfactory product yield, etc., to achieve yield improvement and process The effect of the simple, simplified approach
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 600ml of sterilized water to a 2000ml Erlenmeyer flask, add 10g of sucrose, 120g of dry Saccharomyces cerevisiae, tie the mouth with seven layers of clean gauze, place it in a constant temperature water bath at 30°C for 2 hours, take a sample on a counting plate and count it under a microscope, the number of bacteria Up to 8×10 27 / cm 2 When above, the activation is completed. Add 3000ml of water into the 5L fermenter, heat the jacket to 121°C by introducing 0.3Mpa steam into the jacket and keep it warm for 30 minutes, then cool down to 29°C. Add the activated broth. Weigh 50g of the condensate, dissolve it with 150ml of ethanol at 60°C, and slowly drop it into the fermenter. After the addition is complete, add 2ml of sorbitol. Set the temperature of the fermenter to 29°C, and the air flow rate to 5L / min. After 96 hours of fermentation, take a sample and spot the plate. When the raw material spots completely disappear, the fermentation conversion ends. Use a 300...
Embodiment 2
[0030] Add 600ml of sterilized water to a 2000ml Erlenmeyer flask, add 15g of glucose, 100g of dry Saccharomyces cerevisiae, tie the mouth with seven layers of clean gauze, place it in a constant temperature water bath at 30°C for 3 hours, take a sample on a counting plate and count it under a microscope, the number of bacteria Up to 8×10 27 / cm 2 When above, the activation is completed. Add 3000ml of water into the 5L fermenter, heat the jacket to 121°C by introducing 0.3Mpa steam into the jacket and keep it warm for 30 minutes, then cool down to 29°C. Add the activated broth. Weigh 50g of the condensate, dissolve it with 150ml of ethanol at 60°C, and slowly drop it into the fermenter. After the addition is complete, add 2ml of mannitol. Set the temperature of the fermenter to 29°C, and the air flow rate to 5L / min. After 96 hours of fermentation, take a sample and spot the plate. When the raw material spots completely disappear, the fermentation conversion ends. Use a 300...
Embodiment 3
[0032] Add 600ml of sterilized water to a 2000ml Erlenmeyer flask, add 15g of maltose, 120g of dry Saccharomyces cerevisiae, tie the mouth with seven layers of clean gauze, place it in a constant temperature water bath at 30°C for 5 hours, take a sample on a counting plate and count it under a microscope, the number of bacteria Up to 8×10 27 / cm 2 When above, the activation is completed. Add 3000ml of water into the 5L fermenter, heat the jacket to 121°C by introducing 0.3Mpa steam into the jacket and keep it warm for 30 minutes, then cool down to 29°C. Add the activated broth. Weigh 50g of the condensate, dissolve it with 150ml of ethanol at 60°C, and slowly drop it into the fermenter. After the addition is complete, add 2ml of mannitol. Set the temperature of the fermenter to 29°C, and the air flow rate to 5L / min. After 96 hours of fermentation, take a sample and spot the plate. When the raw material spots completely disappear, the fermentation conversion ends. Use a 300...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com