Human marrow, cord blood or peripheral blood stem cells treating kit and stem cells separating method
A peripheral blood stem cell and umbilical cord blood technology, which is applied to blood/immune system cells, bone/connective tissue cells, animal cells, etc. The effect of low cost and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
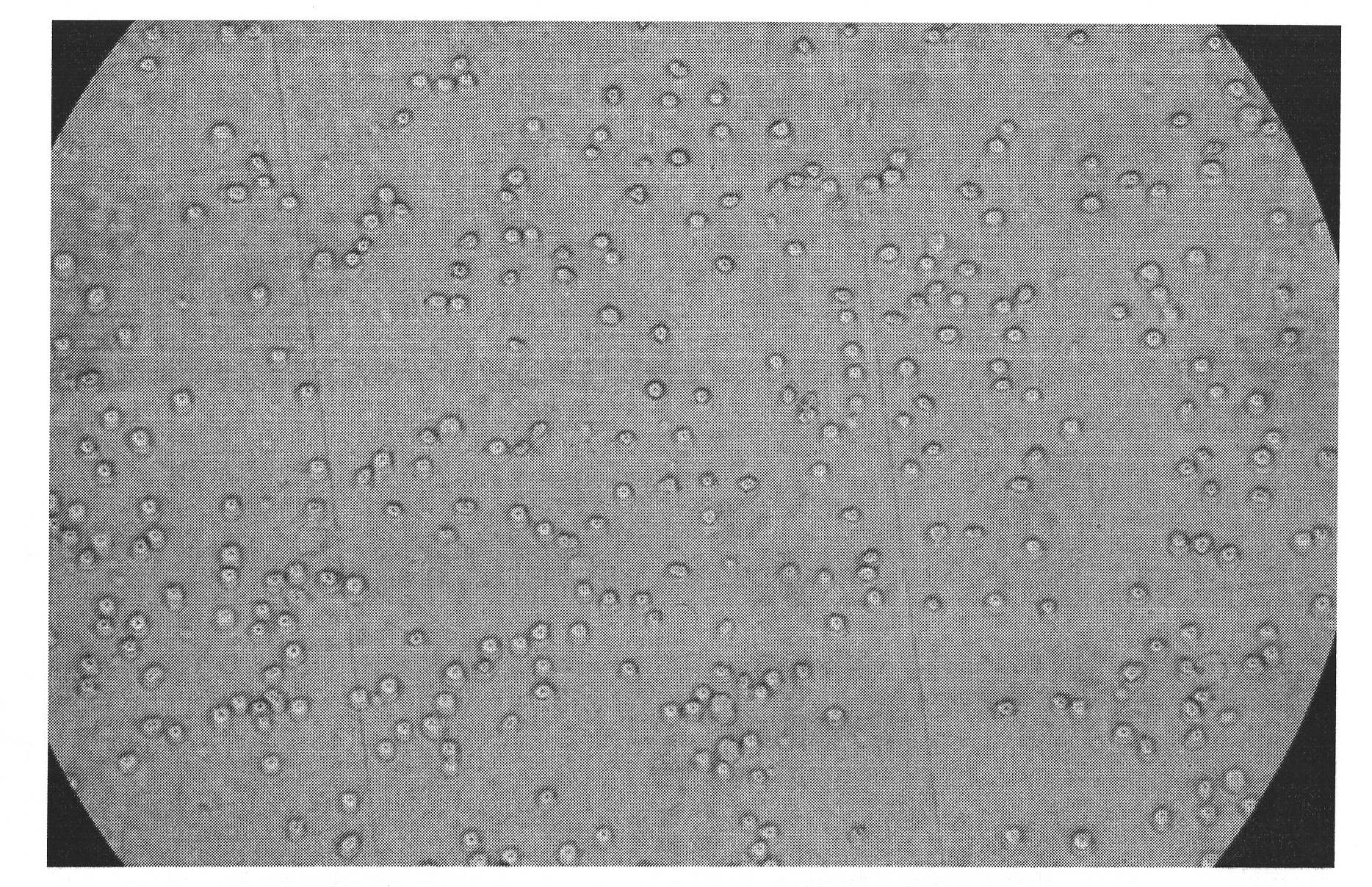
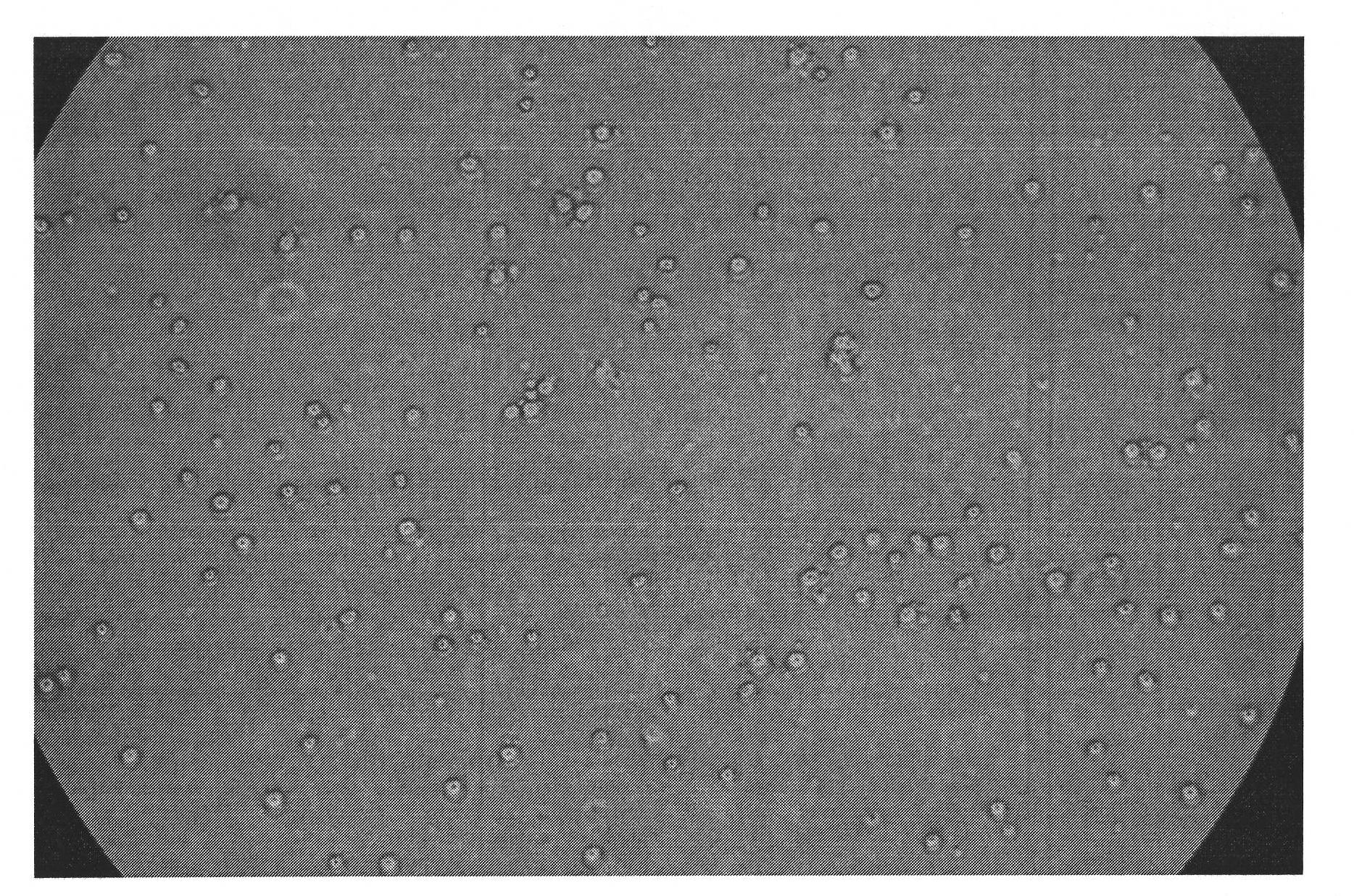
Image
Examples
Embodiment 1
[0069] Isolation of Human Cord Blood:
[0070] Kit composition:
[0071] No. 1 solution: Diluent: PBS solution: 4g sodium chloride, 0.1g potassium chloride, 1.445g disodium hydrogen phosphate 12 water, 0.1g potassium dihydrogen phosphate Dissolve the above reagents with a small amount of water, then add water to 500ml, that is obtained, adjust the pH to 7-7.5 with 5.6% sodium carbonate before use. Add 3% cerebroprotein hydrolyzate (commercially available, prepared as 3% aqueous solution).
[0072] No. 2 solution: Precipitating agent: 6% hydroxyethyl starch (commercially available)
[0073] No. 3 liquid: Separation liquid: make polysucrose and diatrizoate into a separation liquid with a density of 1.075 g / ml.
[0074] The above-mentioned No. 1 and No. 2 liquids were sterilized at 100°C-130°C for 10 minutes-50 minutes, and the endotoxin content was detected to be ≤0.5EU / ml before being bottled. The above-mentioned No. 3 liquid was sterilized at 100°C-130°C for 10 minutes-50 ...
Embodiment 2
[0082] To isolate human bone marrow:
[0083] Kit composition:
[0084] No. 1 solution: Diluent: PBS solution: 4g sodium chloride, 0.1g potassium chloride, 1.445g disodium hydrogen phosphate 12 water, 0.1g potassium dihydrogen phosphate Dissolve the above reagents with a small amount of water, then add water to 500ml, that is To obtain it, adjust the pH to 7-7.5 with 5.6% sodium carbonate before use, and add 3% cerebroprotein hydrolyzate (commercially available, formulated as a 3% aqueous solution).
[0085] No. 2 solution: Precipitating agent: 6% hydroxyethyl starch (commercially available)
[0086] No. 3 liquid: Separation liquid: make polysucrose and diatrizoate into a separation liquid with a density of 1.075 g / ml.
[0087] The above-mentioned No. 1 and No. 2 liquids were sterilized at 100°C-130°C for 10 minutes-50 minutes, and the endotoxin content was detected to be ≤0.5EU / ml before being bottled. The above-mentioned No. 3 liquid was sterilized at 100°C-130°C for 10 m...
Embodiment 3
[0094] Isolation of Human Peripheral Blood:
[0095] Kit composition:
[0096] No. 1 solution: Diluent: PBS solution: 4g sodium chloride, 0.1g potassium chloride, 1.445g disodium hydrogen phosphate 12 water, 0.1g potassium dihydrogen phosphate Dissolve the above reagents with a small amount of water, then add water to 500ml, that is To obtain it, adjust the pH to 7-7.5 with 5.6% sodium carbonate before use, and add 3% cerebroprotein hydrolyzate (commercially available, formulated as a 3% aqueous solution).
[0097] No. 2 solution: Precipitating agent: 6% hydroxyethyl starch (commercially available)
[0098] No. 3 liquid: Separation liquid: make polysucrose and diatrizoate into a separation liquid with a density of 1.075 g / ml.
[0099] The above-mentioned No. 1 and No. 2 liquids were sterilized at 100°C-130°C for 10 minutes-50 minutes, and the endotoxin content was detected to be ≤0.5EU / ml before being bottled. The above-mentioned No. 3 liquid was sterilized at 100°C-130°C f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com