9-hydroxyl-9'-aromatic conjugate substituted fluorene-containing polymer material and preparation and application methods thereof
A polymer material, polymer technology, applied in chemical instruments and methods, luminescent materials, semiconductor/solid-state device manufacturing, etc., to achieve mild conditions and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
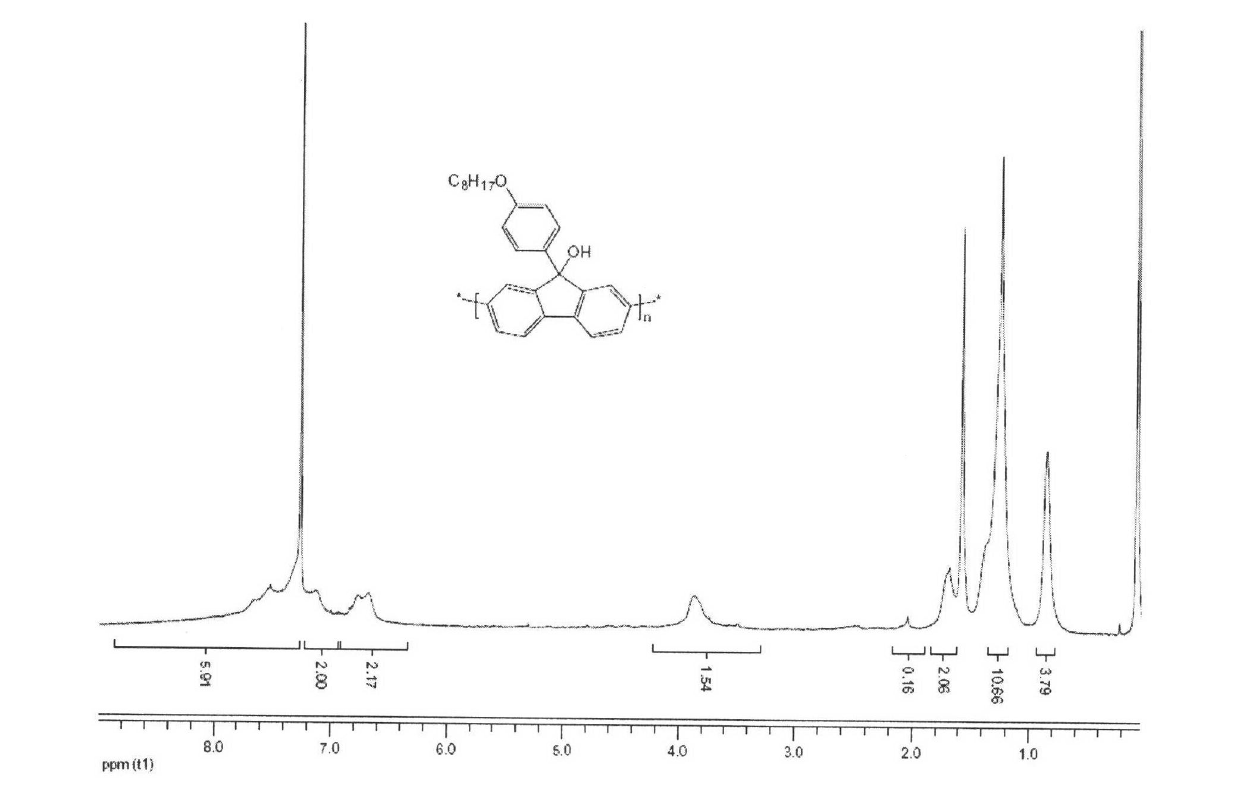
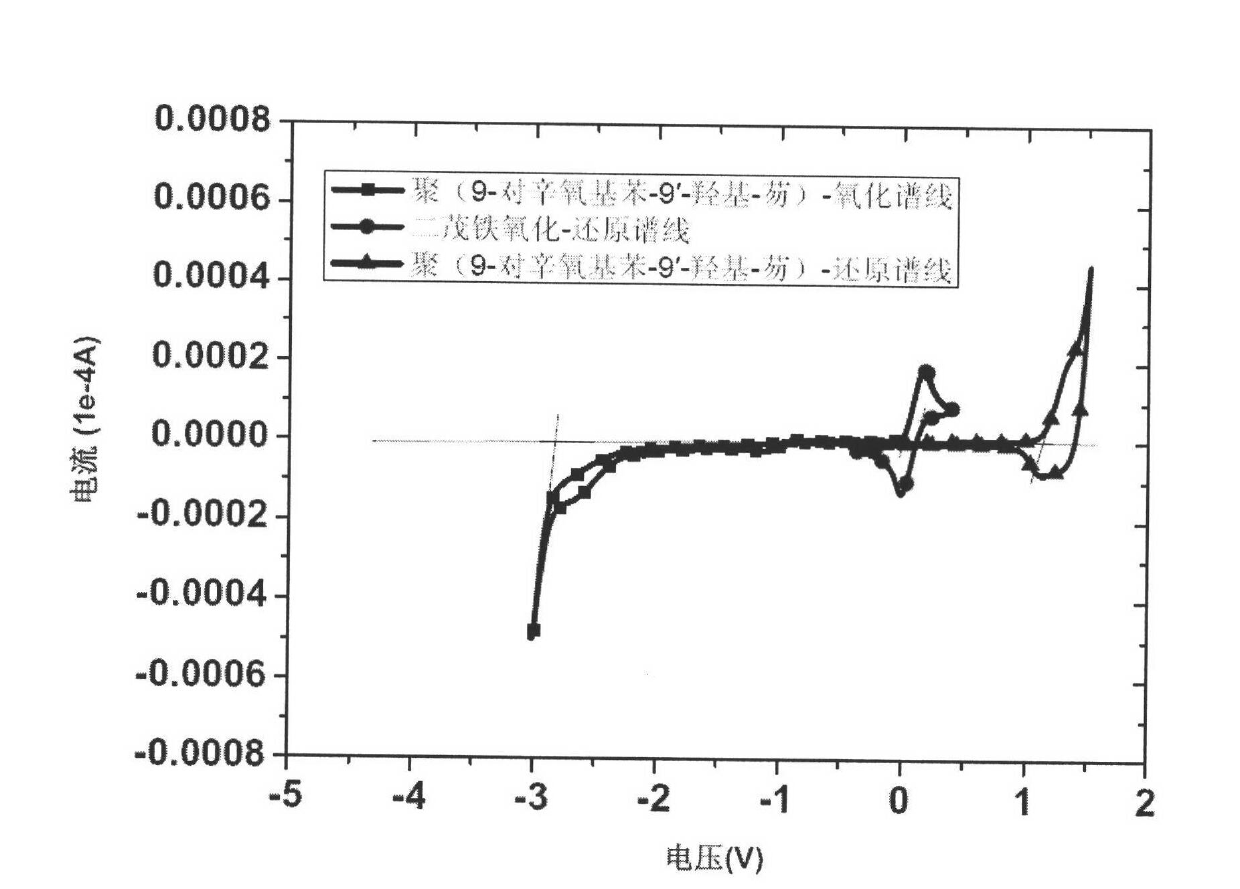
[0041] Embodiment 1, poly(9-octyloxybenzene-9'-hydroxyl-fluorene)
[0042] Poly(9-(4-(octyloxy)phenyl)-9H-fluoren-9-ol)
[0043] p-bromooctyloxybenzene
[0044] 1-romo-4-(octyloxy)benzene
[0045] Experimental procedure: Dissolve p-bromophenol (1 equiv) in acetone, add potassium carbonate and a small amount of tetrabutylammonium bromide, heat to reflux at 70°C, add bromooctane (1.5 equiv) dropwise, react for 24 hours, adjust The pH value was neutral, the liquid was separated with dichloromethane, and passed through the column by rotary evaporation to obtain a transparent liquid (yield 80.5%).
[0046] GC-MS (EI-m / z): 284 (M + ).
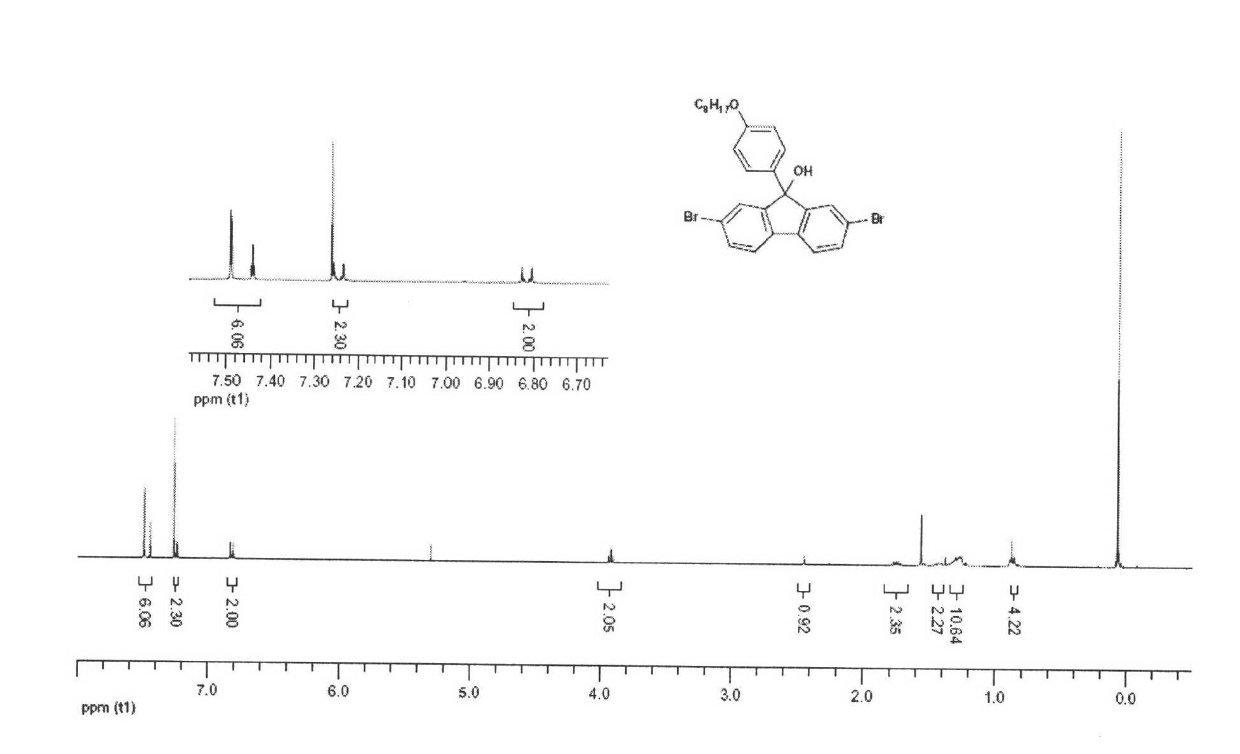
[0047] 2,7-Dibromo-9-p-octyloxybenzene-9′-hydroxyl-fluorene
[0048] 2,7-dibromo-9-(4-(octyloxy)phenyl)-9H-fluoren-9-ol
[0049] Experimental procedure: under the protection of nitrogen, take p-bromooctyloxybenzene (1.2equiv) and magnesium (1.5equiv) (reaction to generate Grignard reagent), and 2,7-dibromo-9-fluorene dissolved in 100ml of tetrahy...
Embodiment 2
[0055] Embodiment 2, poly(9-(3-hexylthiophene)-9'-hydroxyfluorene)
[0056] Poly(9-(4-(octyloxy)phenyl)-9H-fluoren-9-ol)
[0057] 2,7-Dibromo-9-(3-bromothiophene)-9′-hydroxyfluorene
[0058] 2,7-dibromo-9-(3-bromothiophen-2-yl)-9H-fluoren-9-ol
[0059] Experimental procedure: under the protection of nitrogen, add a mixture of tetrahydrofuran (THF), 2,3-dibromothiophene (1.2 equiv) and magnesium ((1.5 equiv)) into the dropping funnel, drop 2d 2 into the flask, 3-dibromothiophene, rapid initiation, ice bath quickly after initiation, then add THF to the flask, slowly add 2,3-dibromothiophene dropwise, after dropwise addition, reflux at 40°C for 2h to prepare lattice reagent. Add 2,7-dibromo-9-fluorenone (1.0 equiv) and THF into another constant pressure dropping funnel, add the prepared Grignard reagent dropwise under ice bath, after the dropwise addition, reflux at 40°C for 24h . with saturated NH 4 Cl quenched the reaction slowly under ice bath, added dichloromethane (DCM)...
Embodiment 3
[0068] Example 3, poly-{[2,7-(9-octyloxybenzene-9'-hydroxyl-fluorene)]-1,6-[pyrene]}
[0069] Poly-{[2,7-(9-(4-(octyloxy)phenyl)-9H-fluoren-9-ol])]-1,6-[pyrene]}
[0070] Experimental procedure: Mix and dissolve 2,7-dibromo-9-p-octyloxybenzene-9'-hydroxy-fluorene (1equiv) and pyrene-1,6-diboronic acid butyl ester (1equiv) in 20ml of toluene and tetrahydrofuran In the mixed solvent, add the catalyst Pd(PPh 3 ) 4 (1-5% of monomer molar weight). Protect from light and pass through nitrogen, then add K 2 CO 3 (2equiv.), react at 90°C for 48 hours, add water after the reaction, use CHCl 3 Extract, dry and concentrate by rotary evaporation, and put into 100mL of methanol. The mixture was suction-filtered, purified by alumina chromatography, concentrated, put into 500ml of methanol, precipitated by suction-filtration, and the collected solid was purified in a Soxhlet purifier for 3 days, re-precipitated in methanol, and dried to obtain a powder product . GPC: M n = 17250; PD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com