Triphenylamine-based hole transmission materials connected by different bridged bonds and preparation method thereof
A hole transport material, triphenylamine-based technology, applied in the field of organic electroluminescence display, can solve the problems of poor film formation of small molecular compounds, unfavorable commercial application, and poor thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
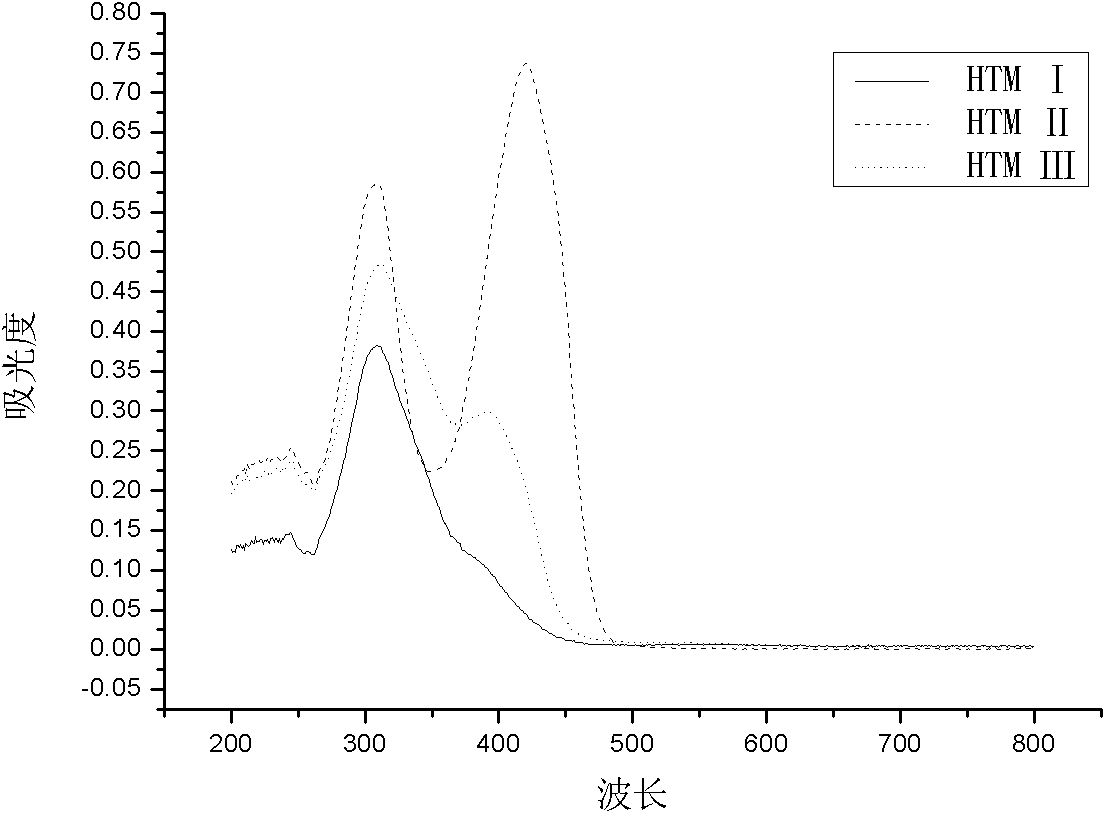
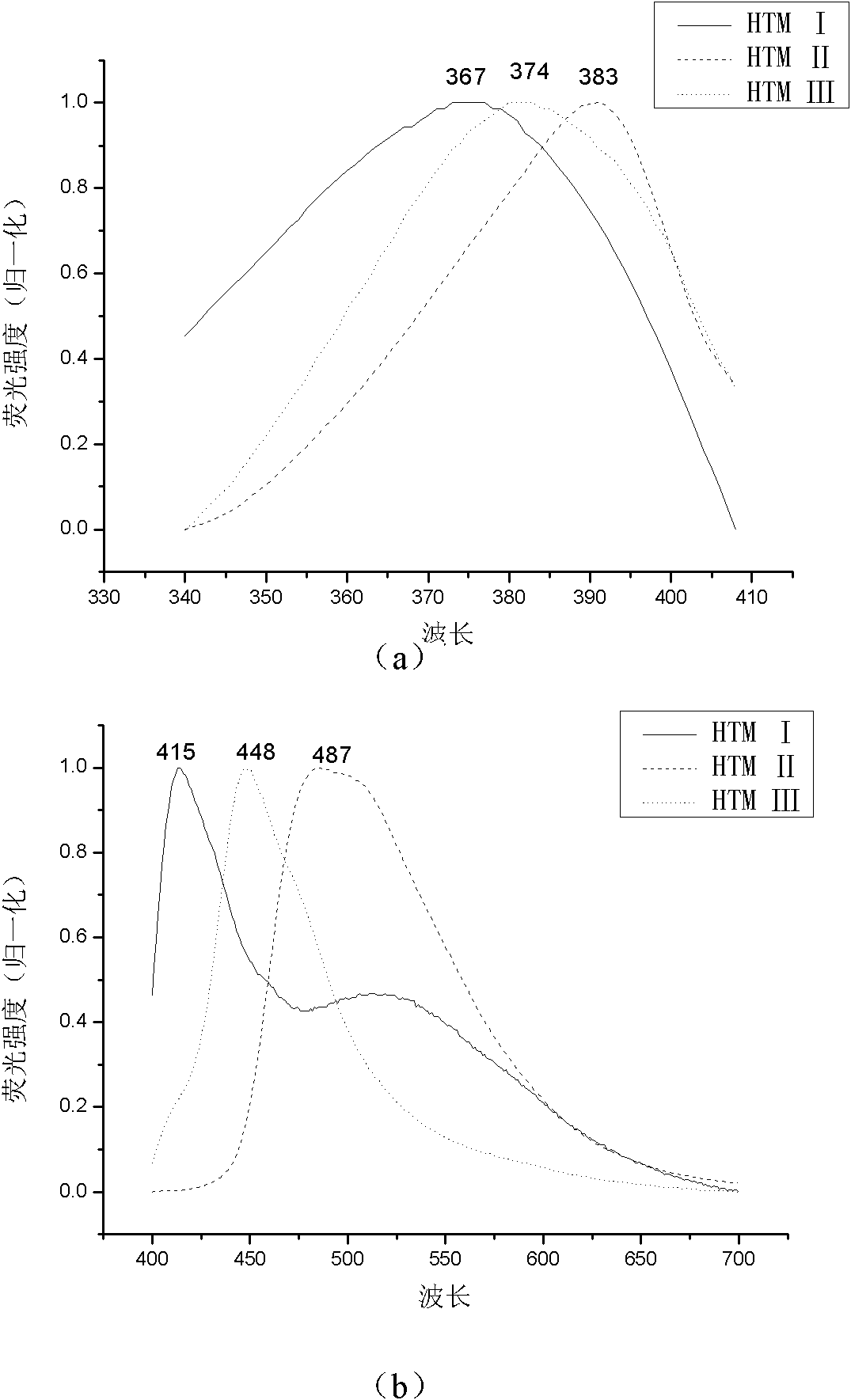
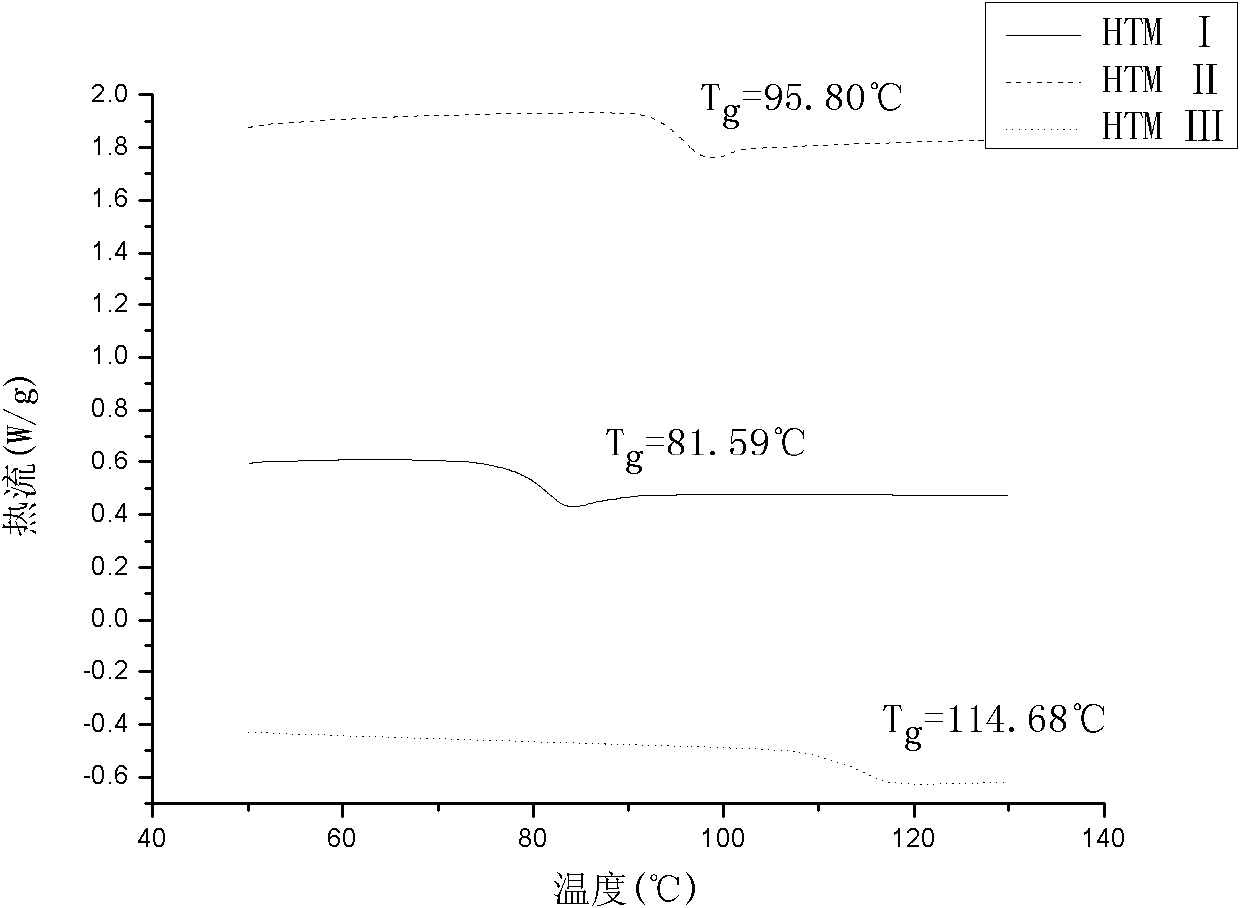
[0026] Examples 1 to 3 are (Z)-1,2-bis(4-N,N-di-p-tolylaminophenyl)ethylene (HTM I)
[0027]
Embodiment 1
[0028] Example 1: Synthesis of (Z)-1,2-bis(4-N,N-di-p-tolylaminophenyl)ethylene (HTM I):
[0029] Under the protection of nitrogen, 3.80g (0.02mol) of titanium tetrachloride and 60mL of tetrahydrofuran were added to a 250mL four-necked flask, 0.48g (0.02mol) of magnesium powder was added with stirring, and the reaction was refluxed for 2 hours. After cooling to room temperature, 0.60g (0.002mol) 4-(N,N-bis(4-methylphenyl)amino)benzaldehyde was added, and the reaction was stirred at room temperature for 8 hours.
[0030] Add 40 mL of 2M hydrochloric acid solution and extract with chloroform to obtain the organic layer. After drying the organic layer with anhydrous magnesium sulfate, the solvent is distilled off under reduced pressure, and the product is dissolved with 50 mL of chloroform, washed with 150 mL of deionized water three times, and collected The organic layer was dried with anhydrous magnesium sulfate, passed through a fast silica gel column (eluent: petroleum ether: ethy...
Embodiment 2
[0031] Example 2: Synthesis of (Z)-1,2-bis(4-N,N-di-p-tolylaminophenyl)ethylene (HTM I):
[0032] Under the protection of nitrogen, 5.70g (0.03mol) of titanium tetrachloride and 60mL of tetrahydrofuran were added to a 250mL four-necked flask, 0.86g (0.036mol) of magnesium powder were added with stirring, and the reaction was refluxed for 3 hours. After cooling to room temperature, 0.60g (0.002mol) 4-(N,N-bis(4-methylphenyl)amino)benzaldehyde was added, and the reaction was stirred at room temperature for 9 hours.
[0033] Add 40 mL of 2M hydrochloric acid solution and extract with chloroform to obtain the organic layer. After drying the organic layer with anhydrous magnesium sulfate, the solvent is distilled off under reduced pressure, and the product is dissolved with 50 mL of chloroform, washed with 150 mL of deionized water three times, and collected The organic layer was dried with anhydrous magnesium sulfate, passed through a fast silica gel column (eluent: petroleum ether: et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com