Easily sublimating medicament injection solution and intravenous injection thereof
A technology for injections and glucose injections, which is applied in the field of easily sublimable drug injection solutions and intravenous injections, can solve the problems of complex operation, complicated rotary steaming process, and low encapsulation rate, and achieve simple preparation process, simple prescription process, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
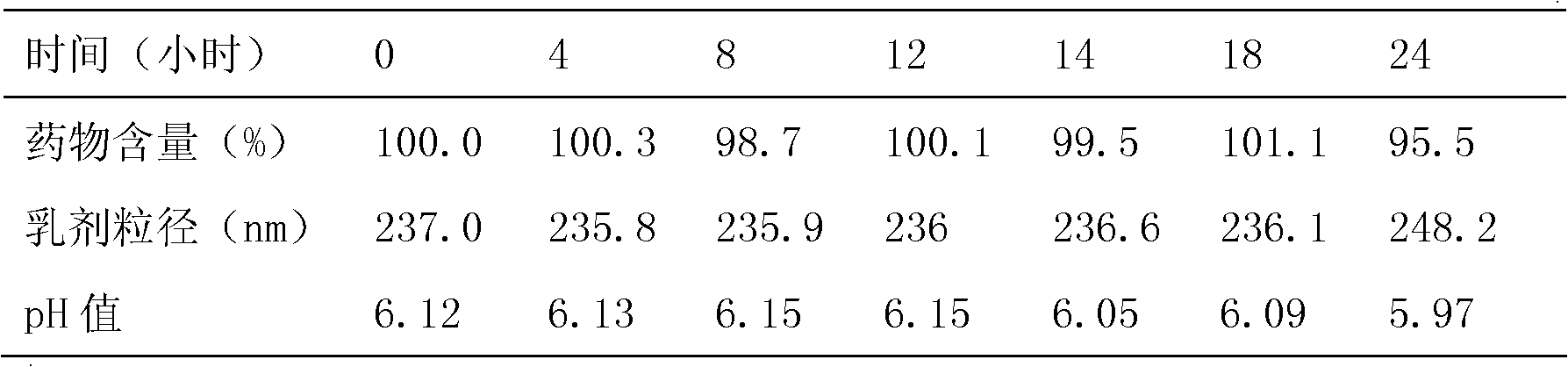
Examples
Embodiment 1
[0041] Embodiment 1 prepares borneol intravenous injection
[0042] 1. Preparation of borneol injection solution:
[0043]Take 5 grams of borneol, add it to 90 ml of polyethylene glycol 400 solvent, stir and dissolve at 75 ° C, then use polyethylene glycol 400 to make up to 100 ml, adjust the pH to 4.5 with an appropriate amount of citric acid, add 0.48 g of needle for Activated carbon, adsorbed at 70°C for 25 minutes, then filtered with a 0.45 μm microporous membrane, divided the filtrate into 2 ml bottles, capped, and then sterilized with high-pressure steam at 105°C for 60 minutes to obtain the borneol injection solution.
[0044] 2. Prepare the emulsion:
[0045] (1) Prepare the oil phase: put 100 grams of soybean oil for injection and 100 grams of caprylic triglyceride in a water bath, heat to 80° C., stir to dissolve, and obtain the oil phase;
[0046] (2) Prepare the water phase: take 650 ml of water for injection, add 12 grams of soybean lecithin for injection, and 2...
Embodiment 2
[0050] Embodiment 2 prepares borneol intravenous injection
[0051] 1. Preparation of borneol injection solution:
[0052] Take 5 grams of borneol, add it to 90 ml of polyethylene glycol 300, stir to dissolve at 80 ° C, then use polyethylene glycol 300 to make up to 100 ml, add 0.02 g of disodium ethylenediamine tetraacetate, adjust with acetic acid When the pH is 4.5, add 0.35 g of activated carbon for needles, absorb at 65°C for 30 minutes, then filter with a 0.45 μm microporous membrane, divide the filtrate into 2 ml bottles, press the cap, and then extinguish it with high-pressure steam at 115°C. Bacteria for 45 minutes to obtain the borneol injection solution.
[0053] 2. Preparation of Emulsion
[0054] (1) Preparation of the oil phase: 100 grams of caprylic triglyceride for injection, 50 grams of soybean oil, 10 grams of lanmenene oil, 10 grams of linseed oil, 15 grams of seabuckthorn oil, 5 grams of zedoary oil, and oyster oil 10 grams, mixed, placed in a water bath...
Embodiment 3
[0058] Embodiment 3 prepares menthol intravenous injection
[0059] 1. Preparation of menthol injection solution:
[0060] Get 0.1 gram of menthol, add it to a mixed solution of 58 milliliters of polyethylene glycol 400, 2 milliliters of absolute ethanol and 25 milliliters of dimethylacetamide, add 0.05 grams of tocopherol, stir and dissolve at 20 ° C, and then Dilute the volume to 100 ml with polyethylene glycol 400, adjust the pH to 5.5 with hydrochloric acid and sodium hydroxide, add 0.26 g of activated carbon for needles, adsorb at 100 ° C for 15 minutes, and then filter with a 0.45 μm microporous membrane. The filtrate was divided into 5 ml bottles, capped, and then sterilized with high-pressure steam at 105° C. for 60 minutes to obtain the menthol injection solution.
[0061] 2. Preparation of emulsion:
[0062] (1) Prepare the oil phase: put 100 grams of soybean oil for injection and 100 grams of caprylic triglyceride in a water bath and heat to 85° C., add 10 grams o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com