Medicinal transparent nano dispersant and preparation method thereof
A nano-dispersion and drug technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of transparent nano-dispersions without drugs, etc., and achieve excellent dissolution effects , easy transportation and storage, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A: Weigh 1g of silybin crude drug and dissolve it in 100mL ethanol;
[0037] B: Weigh 4g polyacrylamide, 1g hydroxypropyl methylcellulose and 0.01g sodium dodecylbenzene sulfonate and dissolve it in 4000mL water, put the beaker with the aqueous solution in an ice water bath, and control the temperature of the aqueous solution at about 3℃ ;
[0038] C: Under stirring conditions of 2000 rpm, pour the bulk drug solution prepared in step A into the aqueous solution in step B to obtain silybin nano-slurry;
[0039] D: Control the spray dryer (SD-Basic, Labplant, UK) with an inlet temperature of 100°C, an outlet temperature of 60°C, a feed rate of 5 mL / min, and a compressed air pressure of 0.6 MPa, and the silybin nano slurry Spray drying to obtain silybin drug compound powder.
[0040] E: Take 110 mg of the composite powder and add it to 1000 mL of water to form a transparent nano-dispersion, in which the drug concentration is 0.1 mg / mL and the average particle size is 75.1 nm.
Embodiment 2
[0042] A: Weigh 1g of silybin crude drug and dissolve it in 20mL ethanol;
[0043] B: Weigh 3.9g polyvinylpyrrolidone and 0.1g sodium dodecylbenzene sulfonate and dissolve in 100mL water, put the aqueous solution in a constant temperature water bath, and control the temperature of the aqueous solution at about 30°C;
[0044] C: Under stirring conditions of 1500 rpm, pour the bulk drug solution prepared in step A into the aqueous solution of step B to obtain silibinin nano-slurry;
[0045] D: Control the spray dryer (SD-Basic, Labplant, UK) with an inlet temperature of 160°C, an outlet temperature of 90°C, a feed rate of 40mL / min, and a compressed air pressure of 0.8MPa. The silibinin nano slurry Spray drying to obtain silybin drug compound powder.
[0046] E: Take 500 mg of the composite powder and add it to 10 mL of water to form a transparent nano-dispersion, in which the drug concentration is 10 mg / mL and the average particle size is 49.3 nm.
Embodiment 3
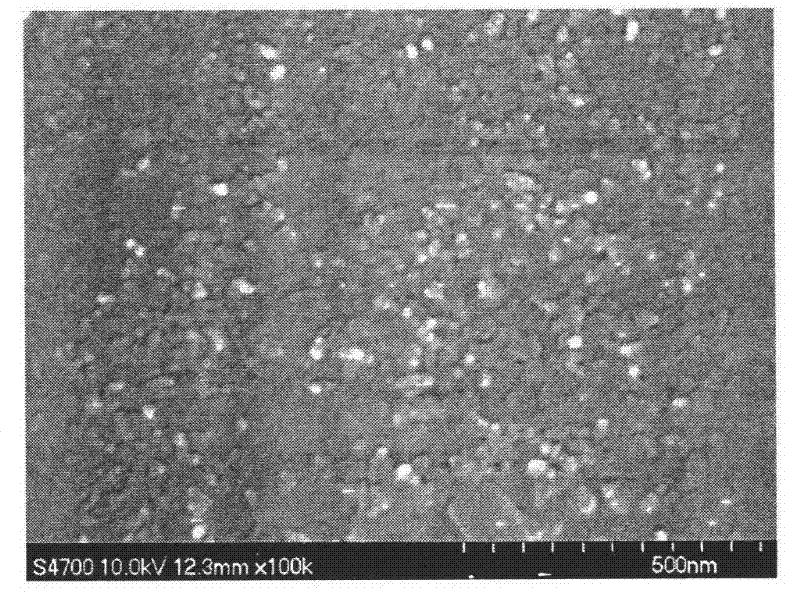
[0048] A: Weigh 1g of silibinin raw material and dissolve it in 50mL of acetone. The scanning electron micrograph of silibinin raw material particles is shown figure 1 , The particle morphology is uneven, and the particle size is very uneven, ranging from 2μm to 40μm;
[0049] B: Weigh 4.95g of polyvinylpyrrolidone and 0.05g of sodium lauryl sulfate and dissolve in 500mL of water, put the aqueous solution in a constant temperature water bath, and control the temperature of the aqueous solution at about 30°C;
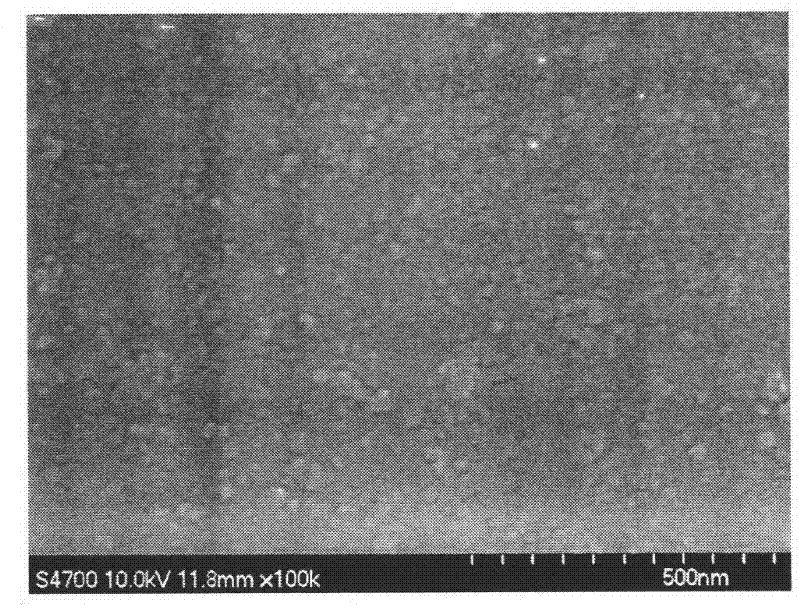
[0050] C: Under the stirring condition of 1000rpm, pour the raw material solution prepared in step A into the aqueous solution of step B to obtain the silibinin nano-slurry. The scanning electron microscope image of the particles in the silibinin nano-slurry is shown figure 2 , The particle morphology is uniform spherical, the particle size is relatively uniform, and the average particle size is 24.3nm;
[0051] D: Control spray dryer (SD-Basic, Labplant, UK) inlet temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com