Preparation method for synthesizing graphene loaded noble metal catalyst in organic phase
A precious metal catalyst, a technology for synthesizing graphite, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve uneven dispersion, large size of metal nanoparticles, and affect the speed of metal nanoparticles nucleation and other problems to achieve the effect of preventing overlapping and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
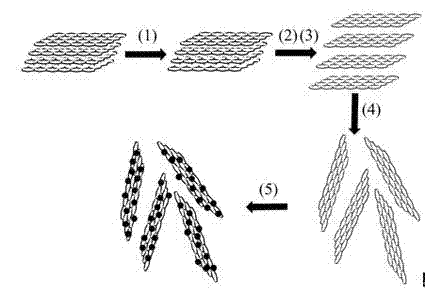
[0032] Such as figure 1 As shown, the preparation method described in this example includes the following steps (precious metal precursor palladium acetylacetonate, stabilizer oleylamine and trioctylphosphine are particularly preferred):
[0033] 1. In a round bottom flask, add 50 mL of concentrated sulfuric acid (92 g), heat to 80-90°C in an oil bath, then add 10 g of potassium persulfate and 10 g of phosphorus pentoxide. 10 g of natural graphite powder was slowly added to the above solution. The mixture was kept in an oil bath at 80-90°C for 4 hours. After cooling to room temperature, the mixture was diluted with deionized water, then vacuum filtered, washed with 3 L of deionized water, and the solid was dried under vacuum for more than one day to finally obtain pre-oxidized graphite powder;
[0034] 2. In a 2L beaker, add 230 mL (4.3 mol) of concentrated sulfuric acid, cool to 0°C, then add 5 g (0.42 mol) of pre-oxidized graphite powder in the first step, and then divide...
Embodiment 2
[0039] 1. In a round bottom flask, add 50 mL of concentrated sulfuric acid (92 g), heat to 80-90°C in an oil bath, then add 16 g of potassium persulfate and 16 g of phosphorus pentoxide. Slowly add 20 g of natural graphite powder into the above solution. The mixture was kept in an oil bath at 80-90°C for 4 hours. After cooling to room temperature, the mixture was diluted with deionized water, then vacuum filtered, washed with 3 L of deionized water, and the solid was dried under vacuum for more than one day to finally obtain pre-oxidized graphite powder;
[0040] 2. In a beaker, add 421 mL (2.15 mol) of concentrated sulfuric acid, cool to 0°C, then add 6 g (0.5 mol) of pre-oxidized graphite powder in the first step, and then add 60 g (0.19 mol) of potassium permanganate in batches Add to the beaker while keeping the temperature below 10°C, then keep in a water bath at 38°C for an optimal time of 2 hours. This mixture was diluted with 0.6L of deionized water, stirred for 2 ho...
Embodiment 3
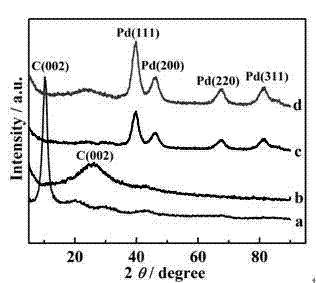
[0045] In a 50 mL round bottom flask, add 20ml of N-methylpyrrolidone solution, under the condition of nitrogen protection, add a certain amount of palladium acetylacetonate to the above solution, and heat it with a heating mantle under the condition of sufficient stirring The optimal reaction temperature is 180-200°C. After 2 hours of reaction, after cooling to room temperature, add 20mL of ethanol to obtain a black precipitate, wash with a large amount of ethanol and acetone, and dry to obtain palladium nanoparticles. For XRD results, see figure 2 (c), the results show that the simple substance of palladium was successfully obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com