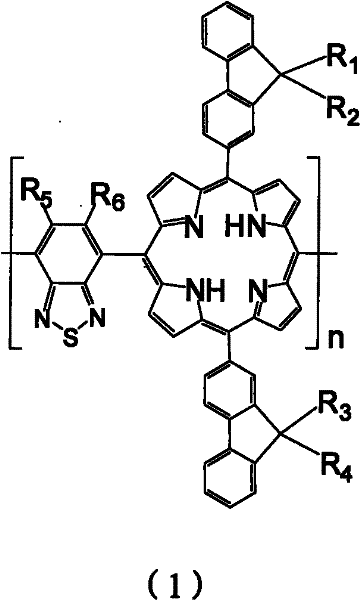
Porphyrin copolymer containing benzothiadiazole unit, its preparation method and application
A technology of benzothiadiazole and copolymers, which is applied in the fields of electrical components, semiconductor/solid-state device manufacturing, and electrical solid-state devices, and can solve problems such as low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
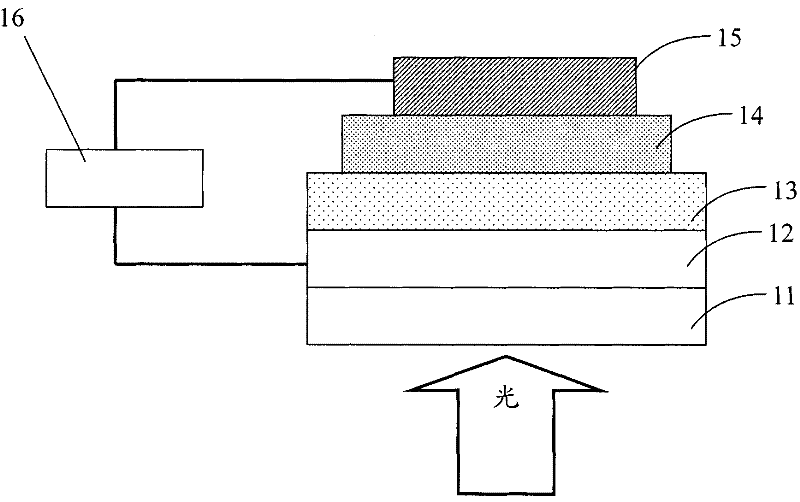
Image
Examples
preparation example Construction
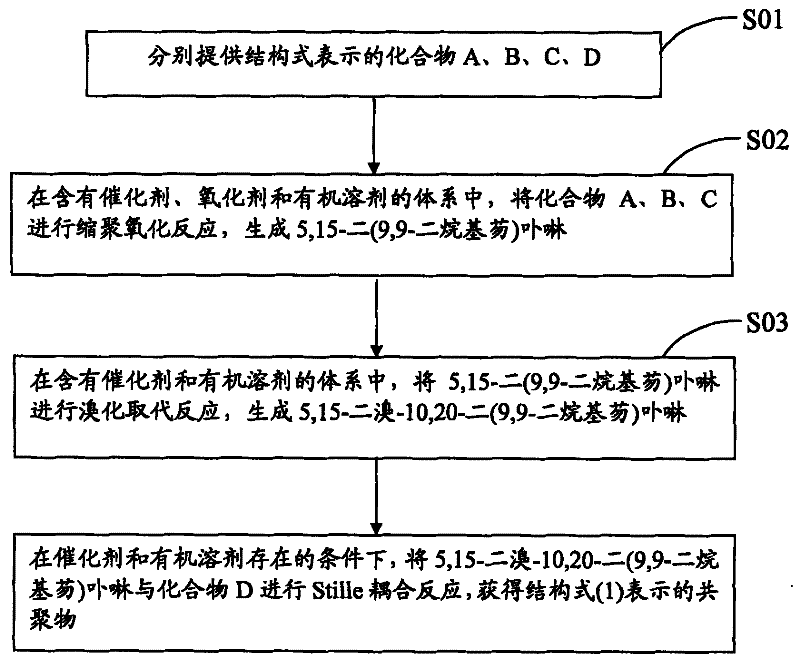
[0034] see figure 1 , the preparation method of above-mentioned porphyrin copolymer containing benzothiadiazole unit comprises the steps:
[0035] S01: respectively provide compounds A, B, C, and D represented by the following structural formulas,
[0036] in,
[0037] R 1 , R 2 , R 3 , R 4 , R 5 , R 6 from C 1 -C 32 the alkyl group;
[0038] S02: In a system containing a catalyst, an oxidizing agent and an organic solvent, the compounds A, B, and C are subjected to a polycondensation oxidation reaction to generate 5,15-bis(9,9-dialkylfluorene)porphyrin;
[0039] S03: In a system containing a catalyst and an organic solvent, 5,15-bis(9,9-dialkylfluorene)porphyrin is subjected to a bromination substitution reaction to generate 5,15-bromo-10,20-bis(9 , 9-dialkylfluorene) porphyrin;
[0040] S04: Under the condition that catalyst and organic solvent exist, carry out Stille coupling reaction with 5,15-dibromo-10,20-two (9,9-dialkylfluorene) porphyrin and compound D, ...
Embodiment 1
[0080] In the copolymer (I) of the present embodiment, R 1 , R 2 , R 3 , R 4 Same as C 8 h 17 , R 5 , R 6 The same as H, its structural formula is as follows:
[0081]
[0082] It can be seen from the structural formula that the porphyrin copolymer containing benzothiadiazole units in Example 1 has a symmetrical bifluorenyl structure, and through this uniform and symmetrical structure, the porphyrin copolymer containing benzothiadiazole units is copolymerized The material has better light-absorbing properties and photoelectric properties. due to R 1 , R 2 , R 3 , R 4 Both use C 8 h 17 , so compounds A and B are both 9,9-dioctyl-2-aldehyde fluorene, so only one kind of raw material is needed, which simplifies the preparation process and reduces the cost, and the yield is high. If it is not such a symmetrical structure, then compounds A and B have different structures, different raw materials need to be obtained, and there are relatively more by-products.
[00...
Embodiment 2
[0101] In the copolymer (II) of this embodiment, R 1 is methyl, R 2 for C 8 h 17 , R 3 for C 10 h 21 , R 4 for C 16 h 33 , R 5 , R 6 The same as H, its structural formula is as follows:
[0102]
[0103] The preparation process is described in detail below.
[0104] Step 1, the preparation of 10-(9-methyl-9-octylfluorene) 20-(9-decyl-9-hexadecylfluorene) porphyrin involves the following reaction formula:
[0105]
[0106] The specific process is as follows: set up an anhydrous and oxygen-free device, weigh the intermediate 9-methyl-9-octylfluorene 0.320g, 9-decyl -9-hexadecylfluorene 0.56g, dipyrromethane 0.30g, dissolved in dichloromethane, blown with nitrogen for 30 minutes, added 2ml of trifluoroacetic acid into the syringe, stirred at room temperature for 3 hours, and then added dichlorodicyano Benzoquinone (DDQ) 1.82g, continue stirring at room temperature for 30 minutes, then add 2ml triethylamine to quench the reaction, concentrate the solvent, filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com