Preparation method of lithium ion phosphate used as positive electrode active material
A positive electrode active material, lithium iron phosphate technology, applied in chemical instruments and methods, battery electrodes, phosphorus compounds, etc., can solve problems such as poor crystal lattice, poor electrical conductivity, large particle size, etc., and achieve uniform particle size distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of positive electrode active material
[0030] 38.08g NH 4 h 2 PO 4 Dissolve in 3100ml of deionized water, stir, add 10.6ml of aniline, fully stir for 20min, 89.46g FeCl 3 ·6H 2O was dissolved in 1450ml of deionized water, and then gradually added dropwise to the above solution, fully stirred for 6 hours, filtered the solution, washed and dried repeatedly with distilled water and ethanol, and the precursor was roasted at 400°C for 4 hours under the protection of Ar atmosphere, and then cooled , Grinding to obtain gray-green powder. The material prepared above is used as the iron source, battery-grade lithium acetate is used as the lithium source, and the molar ratio of the two is 1:1, and 25% sucrose is added as the reducing agent, and absolute ethanol is used as the medium in a high-speed ball mill Ball milling for 2h. The ball-milled samples were dried in a vacuum drying oven at 80°C, and finally baked and heat-treated in a box-type atmosphere fu...
Embodiment 2
[0037] 38.08g NH 4 h 2 PO 4 Dissolve in 3200ml of deionized water, stir, add 15.6ml of pyrrole, fully stir for 20min, dissolve 89.46g of FeCl 3 ·6H 2 O was dissolved in 1650ml of deionized water, and then gradually added dropwise to the above solution, fully stirred for 6 hours, filtered, washed and dried repeatedly with distilled water and ethanol to obtain the precursor, and the precursor was roasted at 400°C for 4 hours under the protection of Ar atmosphere. Cool and grind to obtain powder. The material prepared above is used as the iron source, battery-grade lithium acetate is used as the lithium source, and the molar ratio of the two is 1:1, and 25% sucrose is added as the reducing agent, and absolute ethanol is used as the medium in a high-speed ball mill Ball milling for 2h. The ball-milled samples were dried in a vacuum drying oven at 80°C, and finally baked and heat-treated in a box-type atmosphere furnace. Under the protection of Ar gas, they were kept at 400°C ...
Embodiment 3
[0041] The same method as in Example 1 was used to prepare positive electrode active materials and simulated batteries, except that the iron source was changed to Fe(NO 3 ) 3 .9H 2 O, maintaining its molar ratio to the phosphorus source at 1:1.05.
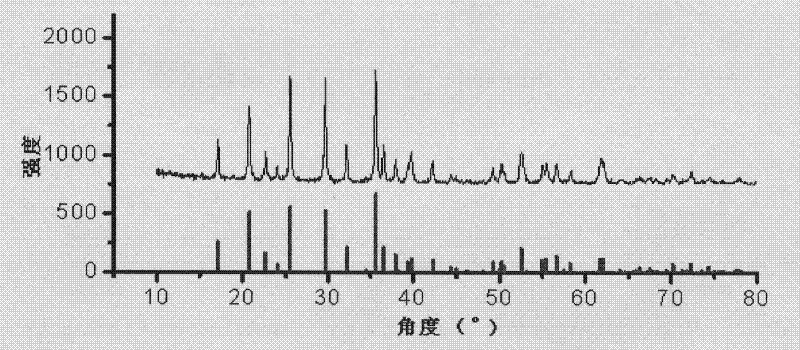
[0042] Using the same method as in Example 1, the particle size of the lithium iron phosphate positive electrode active material can reach about 50nm, and the particle size is in the range of 30-50nm, and the particle size is uniform, the size is basically the same, and there is no agglomeration phenomenon . It can be seen from the XRD figure that the lithium iron phosphate prepared by the present invention has no impurity peaks, pure phase, characteristic peaks (311), (111), and (211) crystal plane diffraction peak intensity is relatively strong, and the half-peak widths are respectively 0.229, 0.249, 0.238, narrower, perfect lattice. The specific surface area (BET) is 46.8m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com