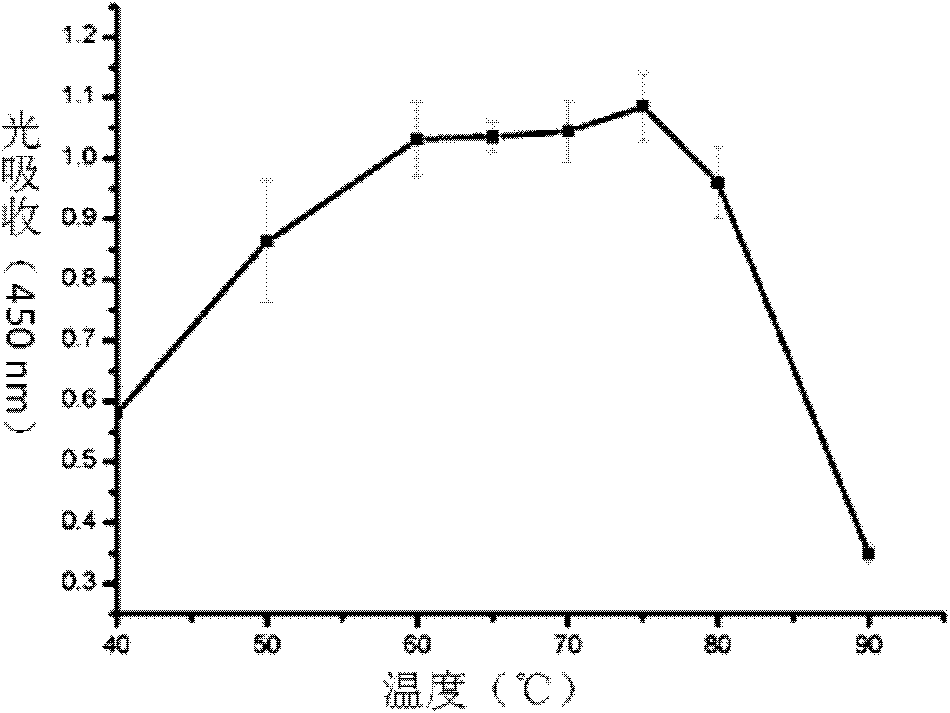
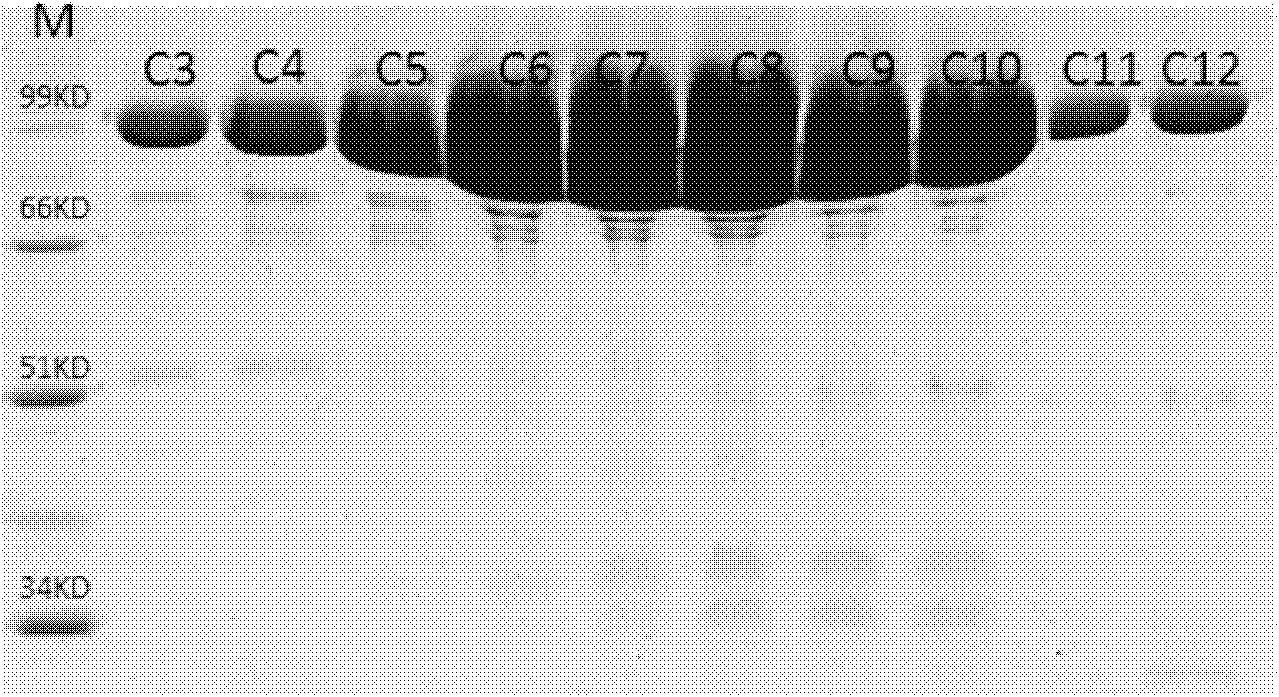Site directed mutagenesis thermoplasma acidophilum F3 factor recombinant protein and application thereof
A recombinant protein and site-directed mutagenesis technology, applied in the biological field, can solve the problems of increased drug screening cost, high production cost of aminopeptidase, poor stability of aminopeptidase, etc., and achieves high yield, simple preparation method and good thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Cloning obtains the mutant gene of Thermoplasma acidophilum F3 factor
[0032] Primers were designed according to the full-length nucleotide sequence of Thermoplasma acidophilum F3 factor published on NCBI, as shown in the following table:
[0033]
[0034] Use the designed pair of primers for PCR amplification, and the reaction system is as follows:
[0035] reaction system:
[0036]
[0037] The PCR reaction conditions are as follows:
[0038] ① Pre-denaturation: 95°C for 5 minutes
[0039] ② Denaturation: 94°C 30s
[0040] ③Annealing: 54℃ 30s
[0041] ④Extension: 72℃ for 2min
[0042] ②-④ cycle 28 times
[0043] ⑤ Final extension: 72°C 10min
[0044] PCR product recovery
[0045] After the PCR, the length of the fragment was analyzed by 1% agarose gel electrophoresis, and the target band was cut out according to the size of the fragment, and the gel cutting product was recovered using the DNA purification kit of Biotech.
Embodiment 2
[0046]Example 2: Transforming the mutant gene into an expression host to obtain a positive expression strain.
[0047] Double digestion reaction of PCR product and plasmid vector
[0048] Enzyme digestion system for PCR products:
[0049]
[0050]
[0051] Reaction conditions: react at 37°C for 2-3h.
[0052] Enzyme digestion system for plasmid vectors:
[0053]
[0054] Reaction conditions: react at 37°C for 6-8h.
[0055] The PCR product and the vector digested product were subjected to 1% agarose gel electrophoresis, and were purified and recovered using a DNA gel recovery kit.
[0056] Connection reaction system:
[0057]
[0058] Mix well and centrifuge for a few seconds, collect the drop on the tube wall to the bottom of the tube, and connect overnight at 16°C.
[0059] Transformation of recombinant plasmids
[0060] (1) Preparation of Competent Cells
[0061] ①Pick a single colony of BL21 (or pick a preserved strain) and inoculate it into about 10ml o...
Embodiment 3
[0083] Example 3: Fermentation and culture of positive expression strains, isolation and purification of point-mutated thermophilic F3 factor recombinant proteins
[0084] Seed culture: Pick positive clones in a conventional method and place them in 5 mL of liquid LB medium containing corresponding antibiotics, and shake and culture at 37°C for 5-6 hours;
[0085] Bacteria expansion culture: Inoculate the seeds into 1L of liquid medium containing the corresponding antibiotics, culture with shaking at 37°C until the bacterial concentration OD600 is 0.8, then cool down to 15°C; add IPTG with a final concentration of 0.6mM after 1 hour, and induce overnight Express.
[0086] Collect the bacteria: 4200rpm, centrifuge at 4°C for 15min, discard the supernatant, and harvest the bacteria; add resuspension solution (25mM Tris-HCl, pH8.0, 100mM NaCl), shake and precipitate the bacteria cells; add protease inhibitor PMSF ( Phenylmethyl sulfonyl fluoride, benzoic acid sulfonyl fluoride) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com