1,2,5-triphenyl substituted pyrrole derivative with gathering induced luminescence performance as well as preparation method and application thereof
A technology of aggregation-induced luminescence and triphenylpyridine, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., to achieve the effects of reducing pollution, simple preparation method, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
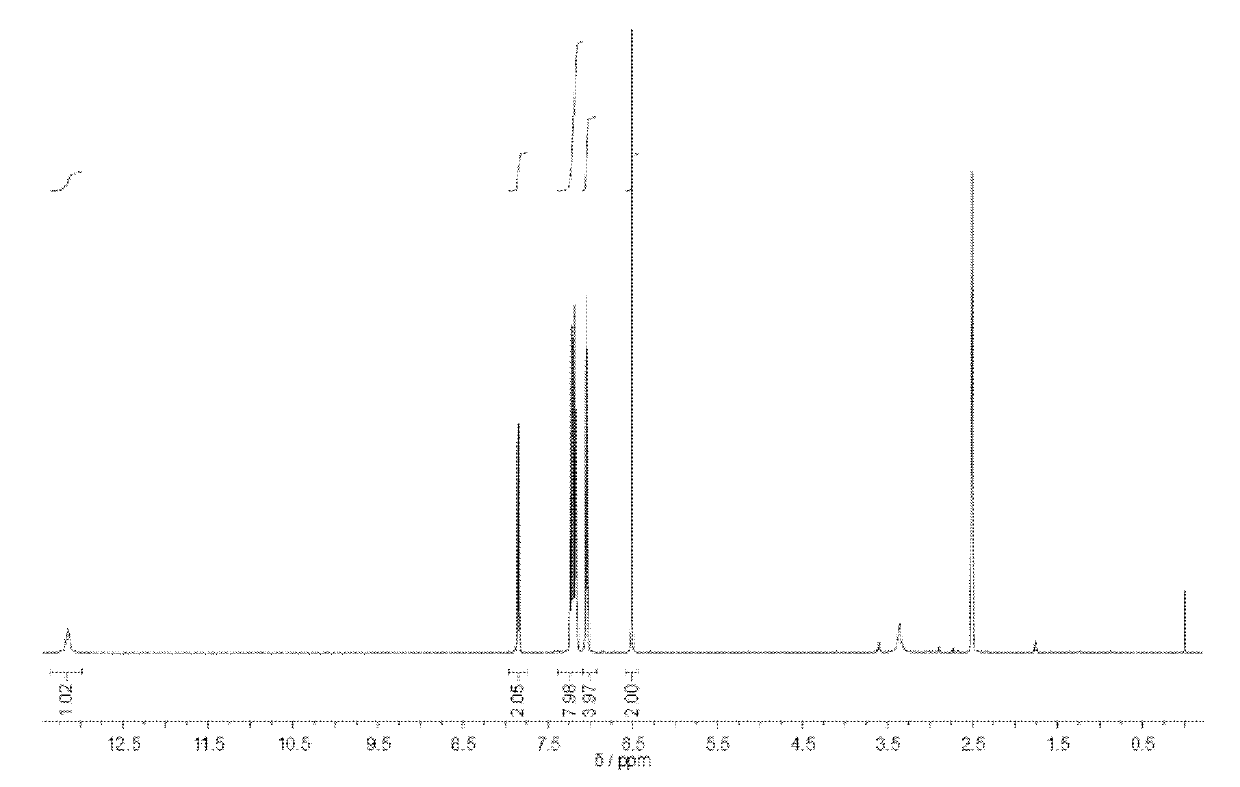
[0047] Preparation of Compound 3: Compound 3 was obtained after acidifying Compound 4 with excess hydrochloric acid.
[0048] The triphenylpyrrole derivatives with aggregation-induced luminescent properties of the present invention can also be prepared by the following synthetic route 2:
[0049] Synthetic route 2:
[0050]
[0051] Wherein the synthesis of compound b is as follows: by triphenylphosphorous palladium dichloride (Pd(PPh 3 ) 2 C1 2 ), triphenylphosphine (PPh 3 ) and cuprous iodide (CuI) in the presence of a catalytic system composed of, in an inert atmosphere, using triethylamine as a solvent and an acid-binding agent, the coupling reaction between methyl p-bromobenzoate and acetylenic alcohol is carried out to prepare the compound b.
[0052] In the preparation reaction of compound b, the catalyst triphenylphosphine palladium dichloride (Pd(PPh 3 ) 2 C1 2 ) to the reactant methyl p-bromobenzoate is preferably 0.001-0.03: 1, more preferably 0.0015-0.01...
Embodiment 1
[0062] Embodiment 1: the synthesis of compound 2
[0063] The synthesis is divided into two steps:
[0064] The first step is the coupling reaction of phenylacetylene: in a 100mL three-necked flask with an airway, add 0.05g (0.5mmol) CuCl, 0.1mL (0.6mmol) N, N, N', N'-tetramethyl ethylenediamine (TMEDA) and 60mL acetone, air was introduced at room temperature for half an hour, after stirring evenly, 4.00g (39.1mmol) phenylacetylene was added, the temperature was raised to 45°C for 8 hours, and the reaction was stopped. Use a rotary evaporator to remove the acetone solvent from the reaction solution, add an appropriate amount of dichloromethane to dissolve it, pour it into a separatory funnel, wash it with 5% hydrochloric acid solution and water in turn, take out the organic phase and use dry anhydrous magnesium sulfate powder to remove water, then evaporate The solvent was dried, and the obtained crude product was recrystallized from ethanol to finally obtain 3.214 g of white...
Embodiment 2
[0066] Embodiment 2: the synthesis of compound 4
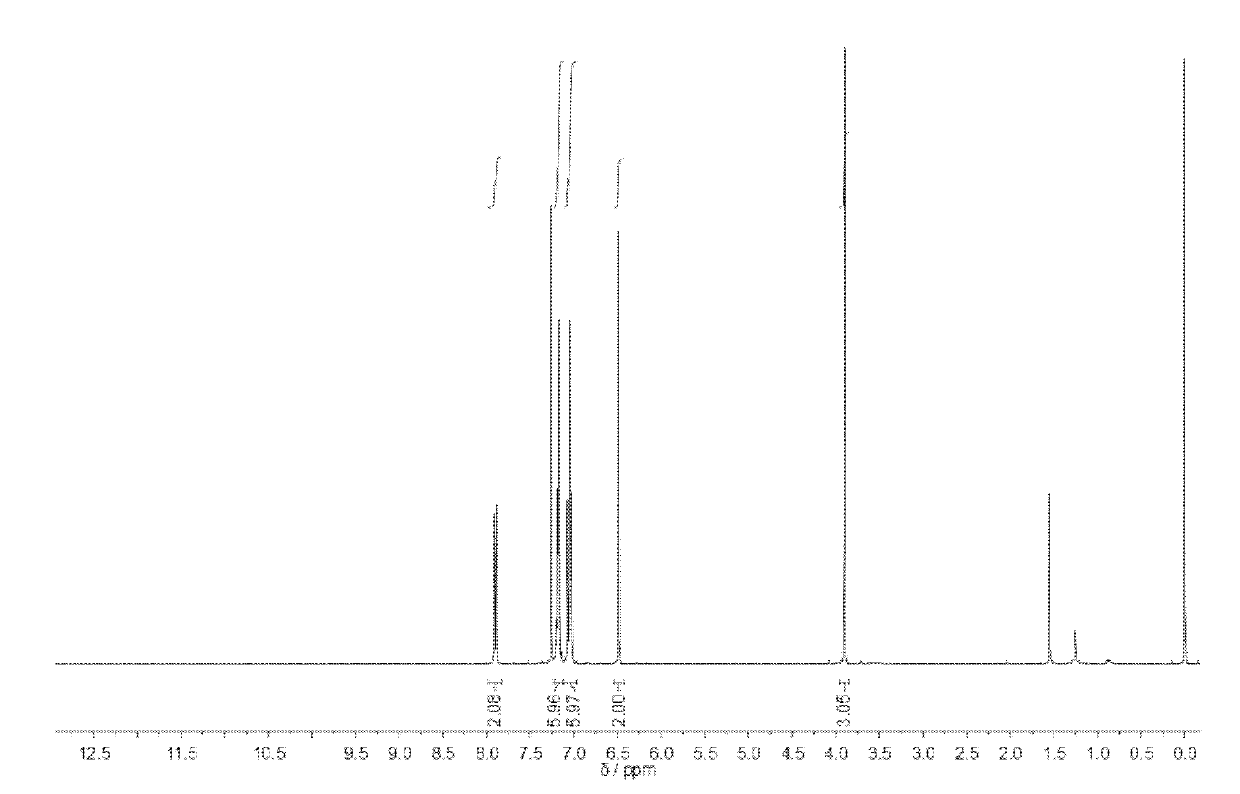
[0067] Add 0.2 g of compound 2 (0.57 mmol) into a 100 ml single-necked bottle, add a small amount of tetrahydrofuran to dissolve, then add an appropriate amount of aqueous sodium hydroxide solution, and reflux at 70°C. After 10 hours, the hydrolysis was complete, and the reaction liquid in the bottle was dropped dropwise into excess HCL aqueous solution, and 0.14 g of the precipitate was collected, which was compound 3 with a yield of 72.5%. figure 2 .
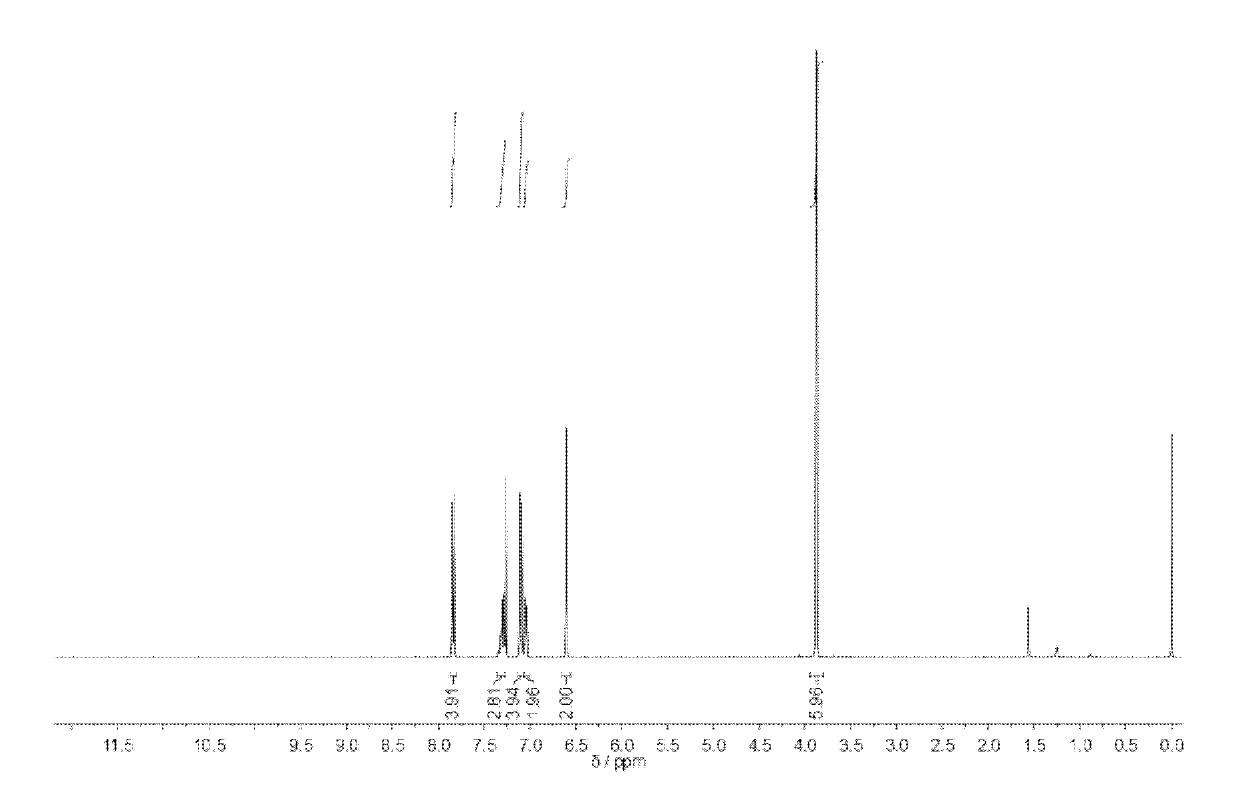
[0068] compound 3 into 10 -3 mol / L methanol solution, and then prepare an equal volume of 10 -3 The mol / L sodium hydroxide methanol solution was mixed with the methanol solution of the above-mentioned compound 3, and stirred for 8 hours to completely neutralize the acid and alkali to obtain the compound 4 with carboxylate anions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com