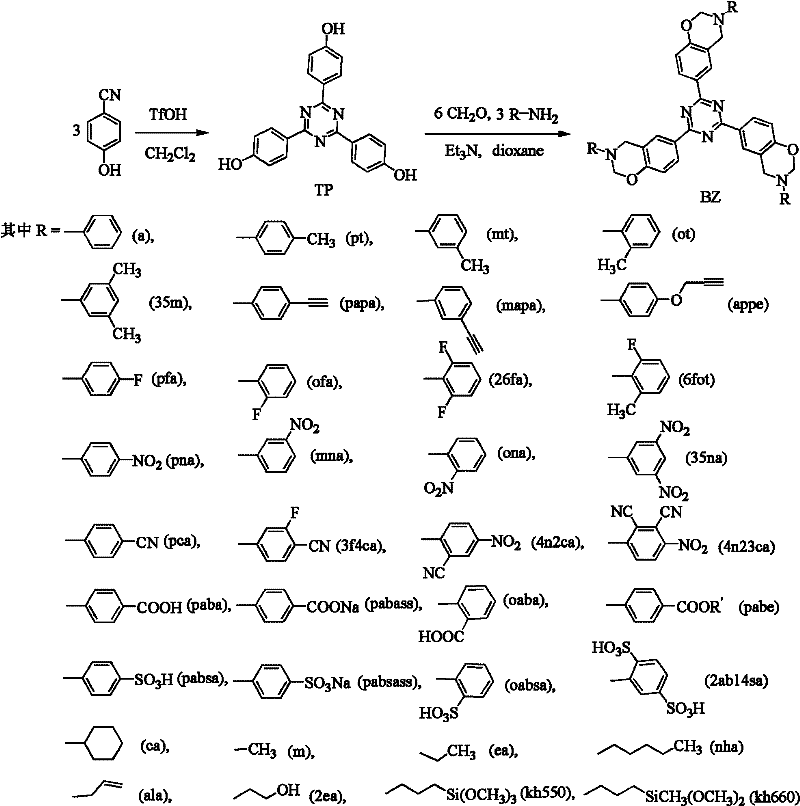
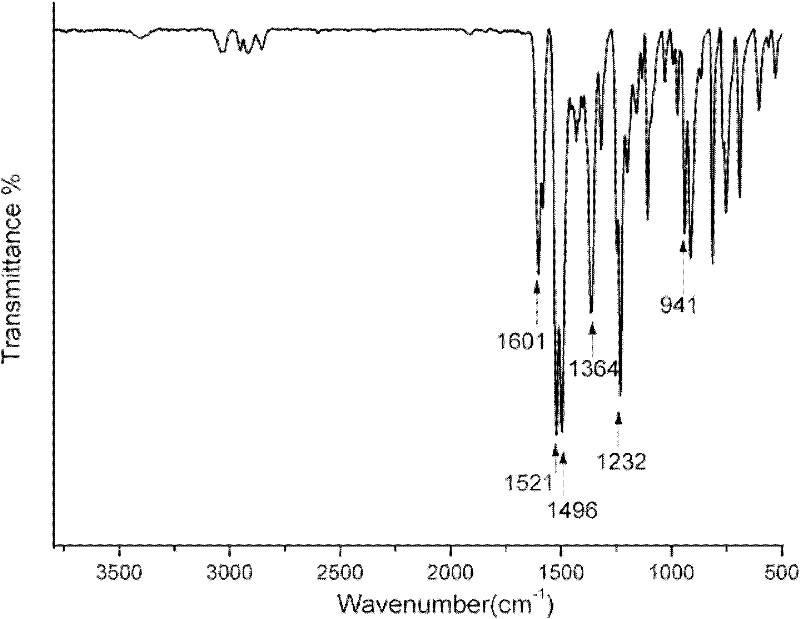
Triazine-containing benzoxazine, triazine-containing benzoxazine polymer, and preparation method thereof
A polycyclic benzoxazine and phenyl technology, applied in the field of polymer materials, can solve the problems of unsatisfactory improvement of cyano group conversion rate, unfavorable performance of polybenzoxazine, and low cyano group conversion rate, etc. Achieve the effects of simple and easy curing process, excellent thermal performance and low curing temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of embodiment 1 triazine-containing polycyclic benzoxazine monomer (BZ-a)
[0050] Synthesis of trihydric phenol (TP) containing triazine ring: add 10.0g p-cyanophenol, 1.8mL trifluoromethanesulfonic acid and 40mL dichloromethane to a 100mL flask successively, and react at room temperature for 2h to obtain a yellow suspension liquid. Wash several times with 1N NaOH aqueous solution and deionized water, let stand to separate layers, take dichloromethane and concentrate to obtain a white solid. Yield 92%.
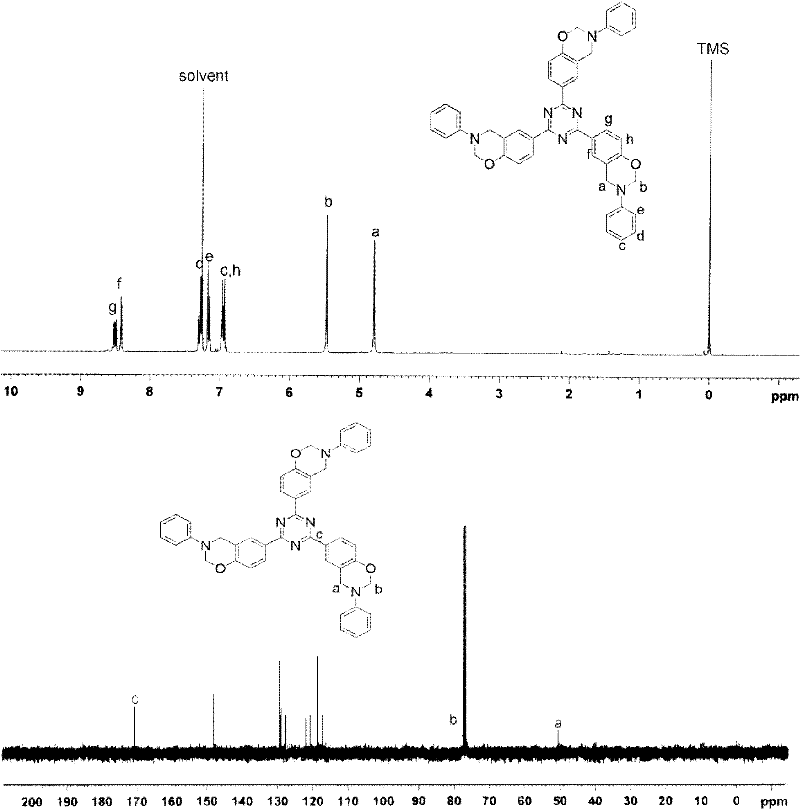
[0051] 1 H NMR (300MHz, CDCl 3 , ppm): 6.97 (d, 6H, Ph-H), 8.55 (d, 6H, Ph-H), 10.29 (s, 3H, Ph-O-H).
[0052] Synthesis of triazine-containing polycyclic benzoxazine monomer (BZ-a): 1.0g TP, 0.504g paraformaldehyde, 0.76mL aniline, 0.5mL triethylamine, 50mL anhydrous Ethanol, stirred and miscible, gradually warmed to reflux, and reacted at reflux temperature for 4 hours to obtain a yellow benzoxazine monomer solution. The reaction mixture was evaporate...
Embodiment 2
[0054] Synthesis of embodiment 2 triazine-containing polycyclic benzoxazine monomer (BZ-a)
[0055] Synthesis of trihydric phenol (TP) containing a triazine ring: 10.0 g of p-cyanophenol, 1.6 mL of trifluoroacetic acid, and 40 mL of dichloromethane were sequentially added to a 100 mL flask, and reacted at room temperature for 3 h to obtain a yellow suspension. Wash several times with 1N NaOH aqueous solution and deionized water, let stand to separate layers, take dichloromethane and concentrate to obtain a white solid. Yield 90%.
[0056] 1 H NMR (300MHz, CDCl 3 , ppm): 6.97 (d, 6H, Ph-H), 8.55 (d, 6H, Ph-H), 10.29 (s, 3H, Ph-O-H).
[0057] Synthesis of triazine-containing polycyclic benzoxazine monomer (BZ-a): 1.0g TP, 1.36mL 37% formaldehyde aqueous solution, 0.76mL aniline, 0.5mL triethylamine, 30mL di Oxyhexane, stirred and miscible, gradually raised the temperature to reflux, and reacted at reflux temperature for 3 hours to obtain a yellow benzoxazine monomer solution...
Embodiment 3
[0058] Synthesis of embodiment 3 triazine-containing polycyclic benzoxazine monomer (BZ-a)
[0059] Synthesis of trihydric phenol (TP) containing a triazine ring: Add 10.0 g of p-cyanophenol, 1.6 mL of trifluoroacetic acid, and 35 mL of chloroform in sequence in a 100 mL flask, and react at room temperature for 2 h to obtain a yellow suspension. Wash several times with 1N NaOH aqueous solution and deionized water, let stand to separate layers, take dichloromethane and concentrate to obtain a white solid. Yield 93%.
[0060] 1 H NMR (300MHz, CDCl 3 , ppm): 6.97 (d, 6H, Ph-H), 8.55 (d, 6H, Ph-H), 10.29 (s, 3H, Ph-O-H).
[0061] Synthesis of triazine-containing polycyclic benzoxazine monomer (BZ-a): 1.0g TP, 1.36mL 37% formaldehyde aqueous solution, 0.76mL aniline, 0.5mL triethylamine, 30mL di Oxyhexane, stirred and miscible, gradually raised the temperature to reflux, and reacted at reflux temperature for 3 hours to obtain a yellow benzoxazine monomer solution. The reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com