Process for separating and purifying recombinant human serum albumin and fusion protein thereof
A human serum albumin and chelation technology, which is applied in the field of purification of human serum albumin, can solve the problems of complicated purification process, high cost, and long cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
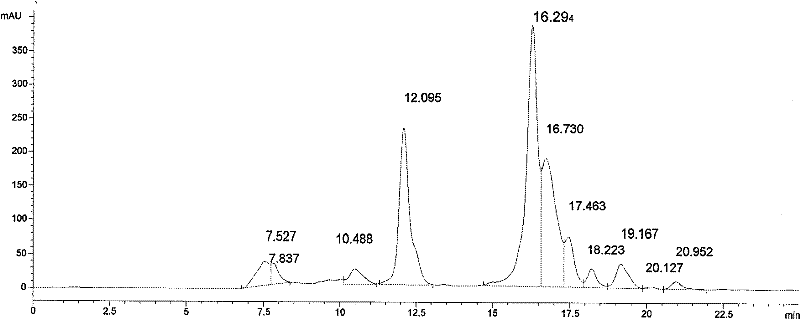
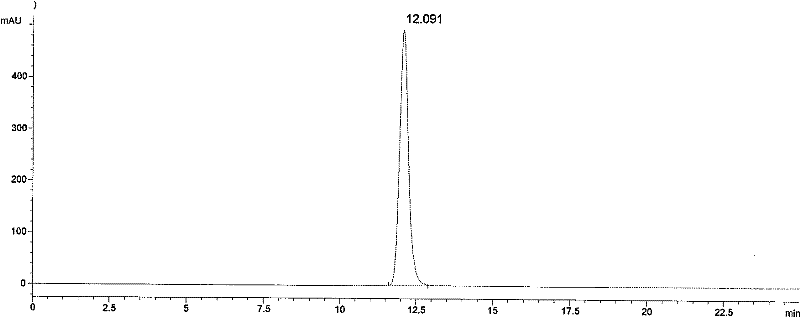
Image
Examples
Embodiment 1
[0065] Step (1) captures the sample using immobilized metal chelate affinity chromatography (IMAC)
[0066] Packing material IMAC Sepharose 6 Fast Flow 1 liter was purchased from GE Company, self-packed chromatographic column, XK50 / 30 column, column diameter 5cm, packing height 15cm, and the preservation solution (20% ethanol) was washed away with purified water.
[0067] Use 0.5 times the column volume of 0.2M copper sulfate solution to pass through the column, and wash away unadsorbed copper ions with purified water.
[0068] Then use buffer A: 20mmol / L phosphate, 600mmol / L sodium chloride, pH7.5, to equilibrate the chromatography column.
[0069] Add phosphate and sodium chloride to the fermentation supernatant containing human serum albumin to make it reach: 20mmol / L phosphate, 600mmol / L sodium chloride, pH7.5.
[0070] Then use AKTA TM The Purify chromatography system was loaded with a flow rate of 50ml / min. After loading, wash with buffer A until the absorbance reache...
Embodiment 2
[0083] Step (1) Use a chromatographic column of a metal chelate chromatographic medium to capture the sample
[0084] The filler Chelating was purchased from GE Company, and the chromatographic column was filled by itself, with a diameter of 5 cm and a packing height of 15 cm. The preservation solution (20% ethanol) was washed away with purified water
[0085] Then pass through the column with 0.5 times the column volume of 0.2M copper sulfate solution, and then wash away the unadsorbed copper ions with purified water.
[0086] Use equilibrium solution A: 20mmol / L phosphate, 600mmol / L sodium chloride, pH7.5 to balance the chromatographic column, and then adjust the sample containing the target protein to the same condition as the buffer solution A, that is, 20mmol / L phosphate, 600mmol / L sodium chloride, pH7.5. Then use AKTA TM The Purify chromatography system was loaded with a flow rate of 50ml / min.
[0087] After sample loading, wash with equilibrium solution A until the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com