Lensing spherical vaterite calcium carbonate crystal with high purity and preparation method thereof
A technology of calcium carbonate and vaterite, applied in the direction of calcium carbonate/strontium/barium, etc., to achieve strong adsorption capacity, easy control of reaction conditions, and good monodispersity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Add 5.299g of sodium carbonate into 100ml of deionized water, stir until completely dissolved, and obtain a solution with a concentration of 0.5mol / L for later use.
[0053] (2) Add 5.549g of calcium chloride into 100ml of deionized water, stir until completely dissolved to obtain a solution with a concentration of 0.5mol / L, and set aside.
[0054] (3) Dissolve 0.242g of 1-butyl-3-methylimidazolium lauryl sulfate in 77ml of deionized water, and stir until completely dissolved. Adjust the pH of the solution to 7 to obtain a solution with a concentration of 7.8mmol / L for subsequent use.
[0055] In this step, the preparation method of 1-butyl-3-methylimidazolium dodecyl sulfate is as follows: Weigh 11.86g (0.085mol) 1-butyl-3-methylimidazolium chloride and 20.32g (0.070mol ) Sodium lauryl sulfate was dissolved in 120ml of dichloromethane, and the resulting solution was placed in a 250ml Erlenmeyer flask and stirred at room temperature for 3 hours. The resulting mixe...
Embodiment 2
[0058] Steps (1) and (2) are as described in Example 1.
[0059] (3) Dissolve 0.323g of 1-butyl-3-methylimidazolium lauryl sulfate in 77ml of deionized water, and stir until completely dissolved. Adjust the pH of the solution to 7 to obtain a solution with a concentration of 10.4mmol / L for subsequent use.
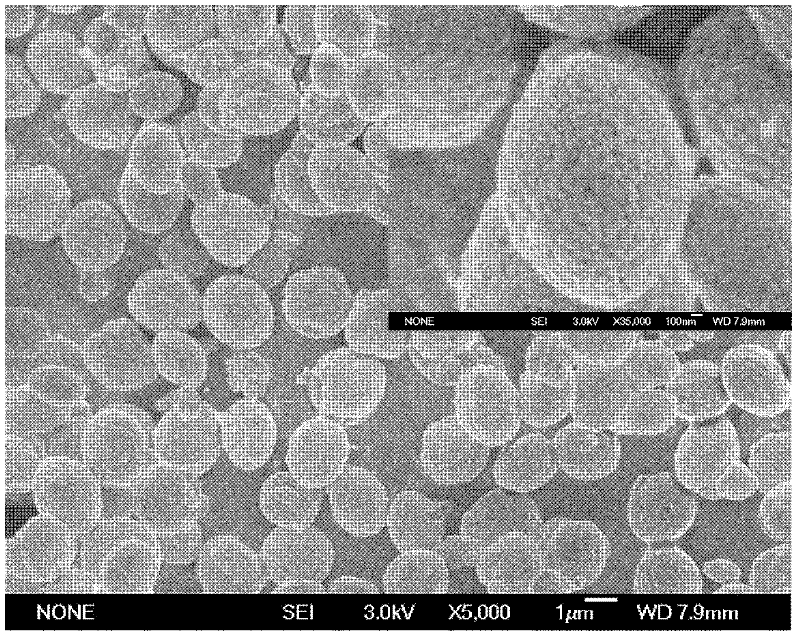
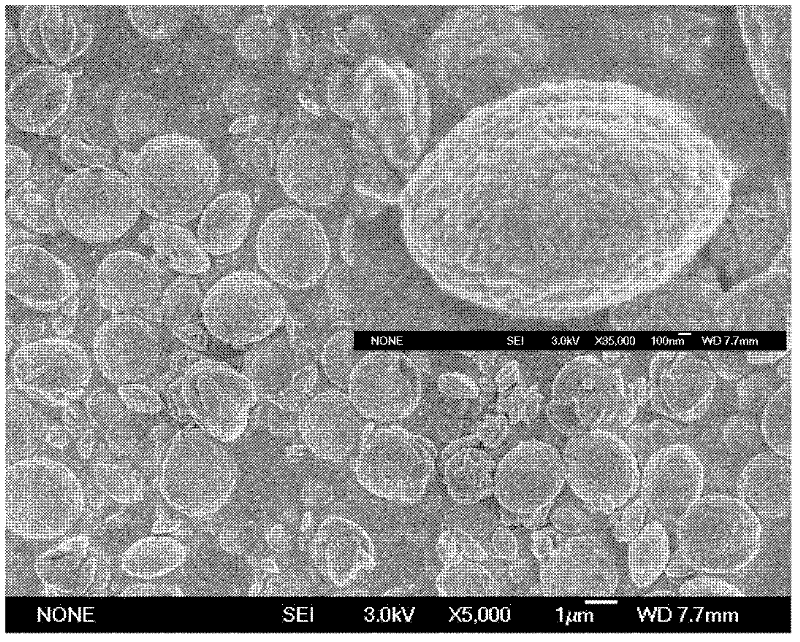
[0060] (4) The preparation process is as described in step (4) in Example 1. Obtain white powdery calcium carbonate, i.e. monodisperse lenticular vaterite type calcium carbonate. The calcium carbonate powder analytical result that obtains is as follows: SEM photo ( figure 2 ) shows that the obtained calcium carbonate is a lens-shaped particle with good monodispersity, with a diameter of about 2.2-2.8 μm and a thickness of about 1-1.5 μm, wherein the nanosphere substructure is spherical nanoparticles with a size of about 100-130 nm. From Figure 5 According to the X-ray powder diffraction results, there is no miscellaneous peak in the spectrum, and the product is pure v...
Embodiment 3
[0062] Steps (1) and (2) are as described in step (1) (2) in Example 1.
[0063] (3) Dissolve 0.484g of 1-butyl-3-methylimidazolium lauryl sulfate in 77ml of deionized water, and stir until completely dissolved. Adjust the pH of the solution to 7 to obtain a solution with a concentration of 15.6mmol / L for subsequent use.
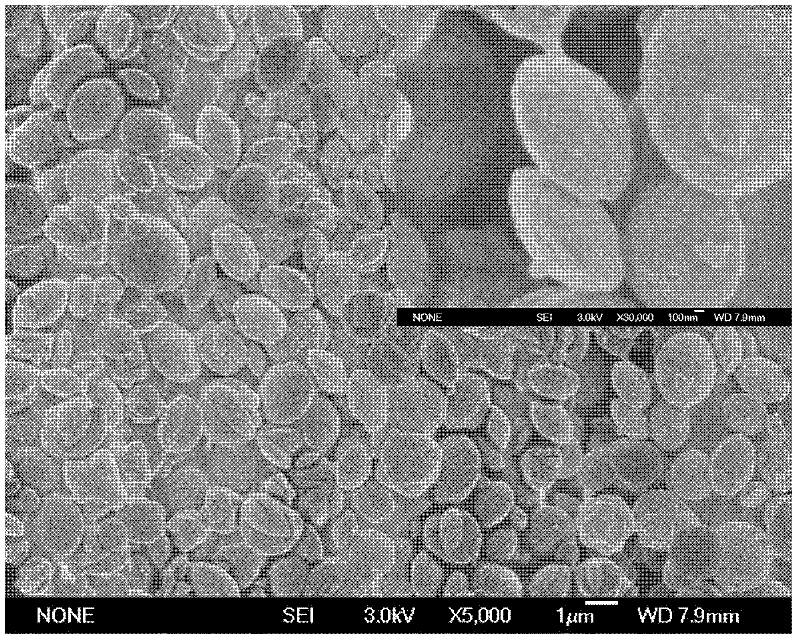
[0064] (4) The preparation process is as described in step (4) in Example 1. Obtain white powdery calcium carbonate, i.e. monodisperse lenticular vaterite type calcium carbonate. The calcium carbonate powder SEM result that obtains is similar to the result of embodiment 2. From Figure 5 According to the X-ray powder diffraction results, there is no miscellaneous peak in the spectrum, and the product is pure vaterite.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com