The preparation method of latamoxef sodium
A technology of laoxef sodium and p-methoxybenzyl, applied in the field of laoxef sodium, can solve the problems of increasing equipment investment, increasing post-processing steps, affecting production efficiency, etc., and achieves reducing equipment investment, reducing energy consumption, The effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
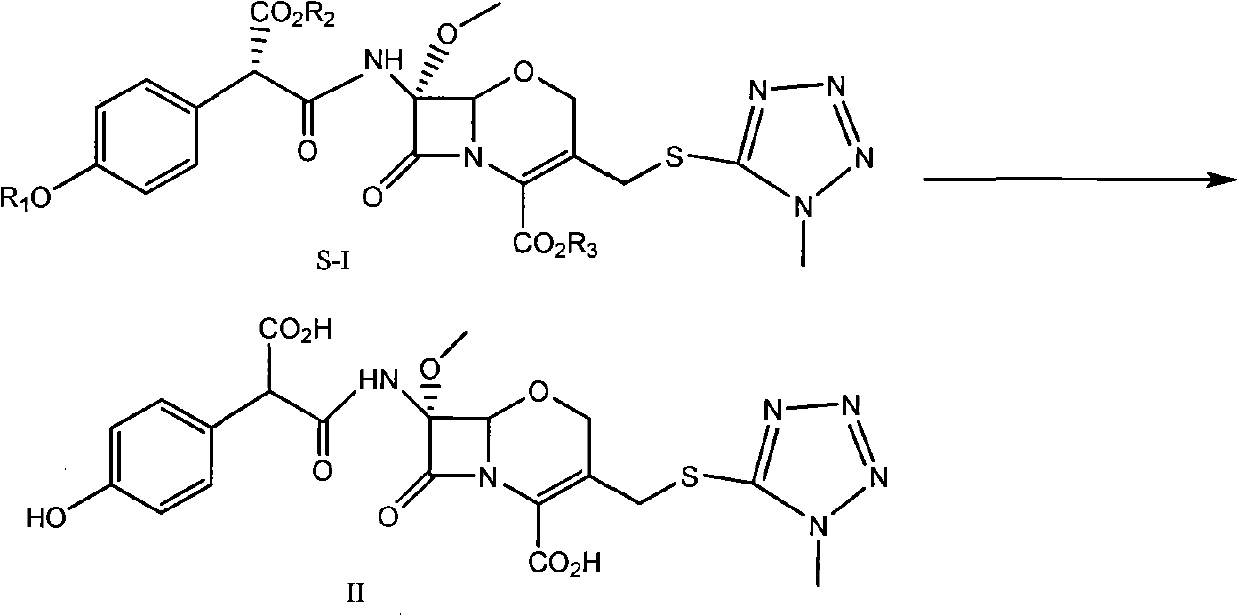
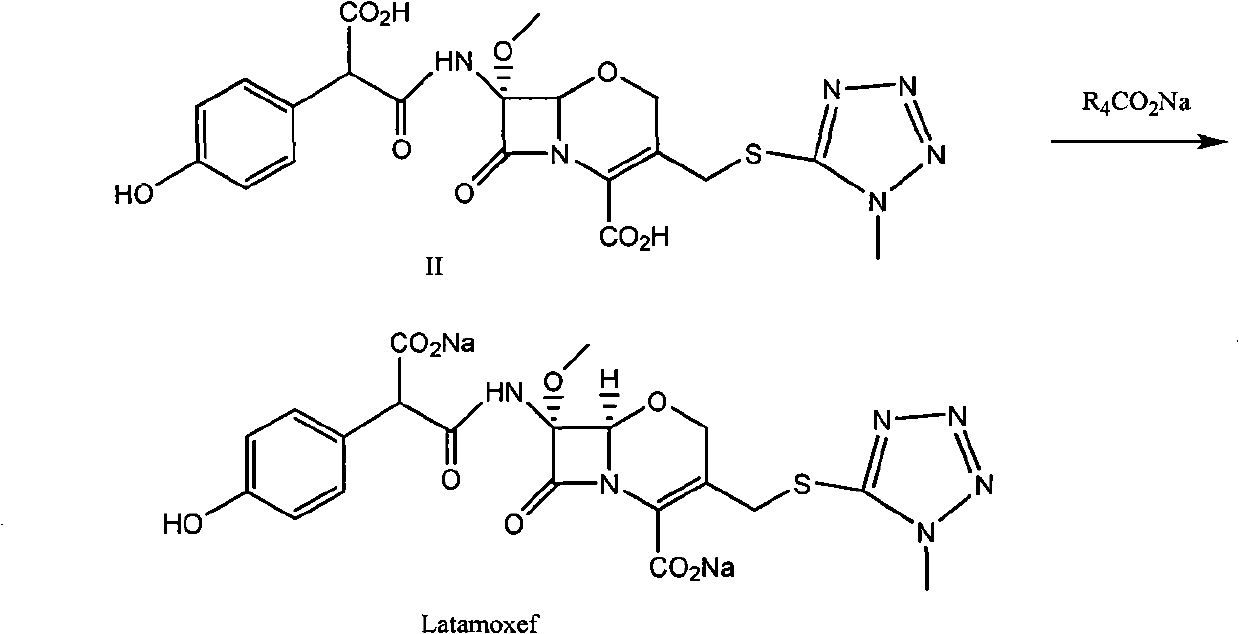
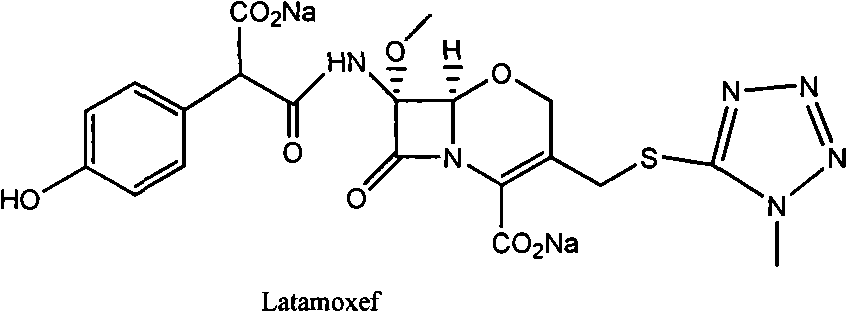
[0052] Compound (S)-I (R1=p-methoxybenzyl, R2=p-methoxybenzyl, R3=benzhydryl) (3.5Kg) was dissolved in dichloromethane (2L), cooled to 0°C , add anisole (15L), dropwise add trifluoroacetic acid (3.5Kg), keep warm at 0°C for 1.0h, add dropwise methyl tert-butyl ether (20L), stir, filter, and dissolve the solid with acetone (20L), Cool to 0°C, add sodium isooctanoate (1.9Kg) / acetone (30L) dropwise, after the addition is complete, react for 30min, filter, dissolve the solid in deionized water (8L), wash with isopropyl acetate (5L), and Decolorized with activated carbon, filtered, and freeze-dried to obtain Latamoxef Sodium. Yield: 87.5%.
Embodiment 2
[0054] Compound (S)-I (R1=H, R2=p-methoxybenzyl, R3=benzhydryl) (3Kg) was dissolved in dichloromethane (5L), cooled to -20°C, and anisole was added (8L), add trifluoroacetic acid (13Kg) dropwise, keep warm at -20°C to -15°C for 1.0h, heat up to 0°C to 15°C for 1.0h, recover dichloromethane and trifluoroacetic acid under reduced pressure, add diethyl ether ( 20L), stirred, filtered, dissolved the solid with methanol (6L), cooled to -5~10°C, added dropwise sodium isooctanoate (1.4Kg) / tetrahydrofuran (60L), after the dropwise addition was completed, reacted for 30min, filtered, and the solid was dissolved in In deionized water (10 L), washed with ethyl acetate (5 L), decolorized with activated carbon in the aqueous phase, filtered, and lyophilized to obtain Latamoxef sodium. Yield: 90%.
Embodiment 3
[0056] Compound (S)-I (R1=H, R2=p-methoxybenzyl, R3=benzhydryl) (3Kg) was dissolved in dichloromethane (10L), cooled to -30°C, and anisole was added (3L), add trifluoroacetic acid (18Kg) dropwise, and slowly heat up to -10°C~0°C for 1.5h after dropping, add isopropyl ether (50L) dropwise, stir, filter, and dissolve the solid with ethyl acetate (25L) , cooled to 0 ~ 10 ° C, dropwise added sodium isooctanoate (1.4Kg) / ethyl acetate (20L), after the dropwise addition was completed, reacted for 30min, filtered, the solid was dissolved in deionized water (10L), butanone (5L) Wash, decolorize with activated carbon in the aqueous phase, filter, and freeze-dry to obtain Latamoxef Sodium. Yield: 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com