Preparation method of broad-spectrum long-acting penicillin antibiotic ticarcillin sodium
A technology of ticarcillin sodium and ticarcillin sodium, which is applied in the field of preparation of spectral long-acting penicillin antibiotic ticarcillin sodium, can solve problems such as difficult control, cumbersome steps, and difficult hydrolysis reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
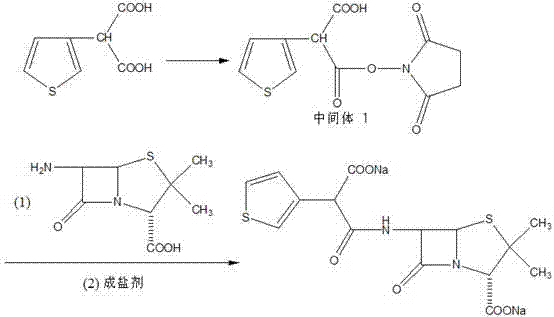
[0026] Dissolve 3-thiophenemalonic acid (0.74g, 4.0mmol) in 10ml of ethanol, add DMAP (730mg, 6mmol) and DCC (1.2g, 6.0mmol) and stir at room temperature for half an hour, then add N-hydroxysuccinimide (0.47g, 4mmol), stirred at room temperature for half an hour, added saturated NH4Cl solution and stirred, extracted with ethyl acetate, separated the ethyl acetate layer, washed the organic phase with saturated brine, dried over anhydrous sodium sulfate, evaporated the solvent, and Eluted by chromatography (ethyl acetate / methanol=10:1), 1.05 g of Intermediate 1 was obtained with a yield of 93%.
[0027]
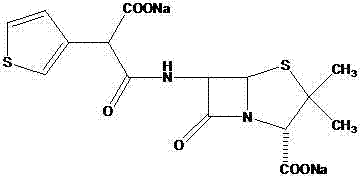
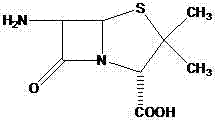
[0028] Dissolve Intermediate 1 (1.05g, 3.7mmol) in 20ml of dichloromethane, add 6-APA (0.8g, 3.7mmol) and 10ml of triethylamine and stir at room temperature for 8 hours, adjust with 1mol / l hydrochloric acid solution Acidic (PH=2.0), and react at -5°C for 2h. Extract with dichloromethane, decolorize with activated carbon, dry and concentrate to obtain 1.35 g of ticarcillin, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com