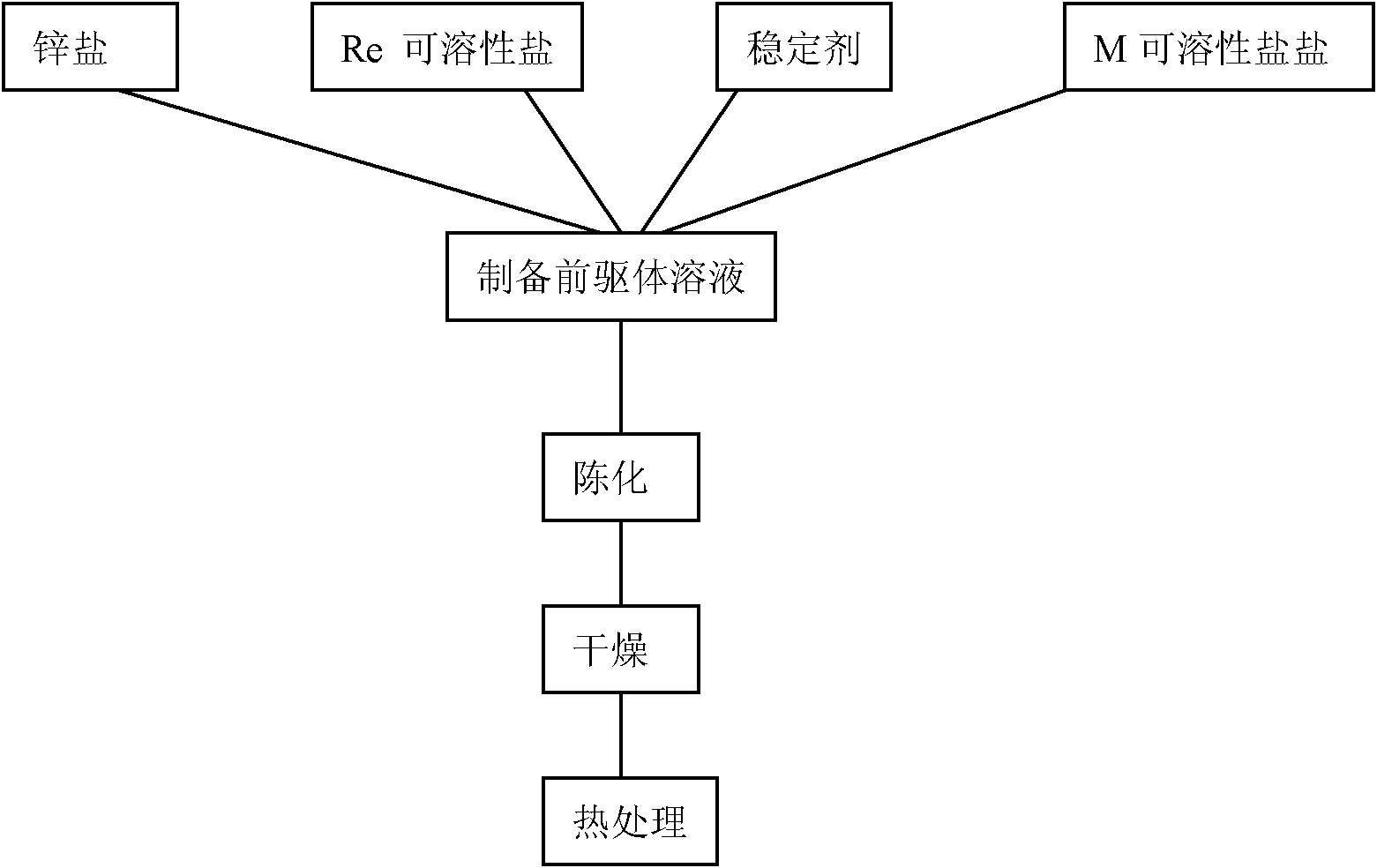
Zinc oxide based fluorescent powder and preparation method thereof
A zinc oxide-based, phosphor technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of low luminous intensity of luminescent materials, and achieve the effects of short preparation period, easy production conditions, and few process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 0.3398g of chloroauric acid and dissolve it in water to make 1000mL of chloroauric acid solution with a concentration of 0.001mol / L.
[0033]Take by weighing 0.4939g of zinc acetate and 0.1115g of europium nitrate respectively, measure 0.16mL of monoethanolamine and 2.5mL of chloroauric acid solution, and use ethylene glycol methyl ether as a solvent to prepare 50mL of zinc and europium solutions containing 0.05mol / L. After stirring in a water bath at 50°C for 6 hours, a uniform precursor solution was obtained, and then the obtained precursor solution was aged in an oven at 75°C for 75 hours to obtain Zn 0.90 Eu 0.10 O: 0.001 Au colloid.
[0034] Dry the colloid obtained in the previous step in an oven at 100°C for 90 hours, and then place it in a corundum crucible at 600°C for 6 hours to obtain Zn 0.90 Eu 0.10 O: 0.001Au zinc oxide-based phosphor.
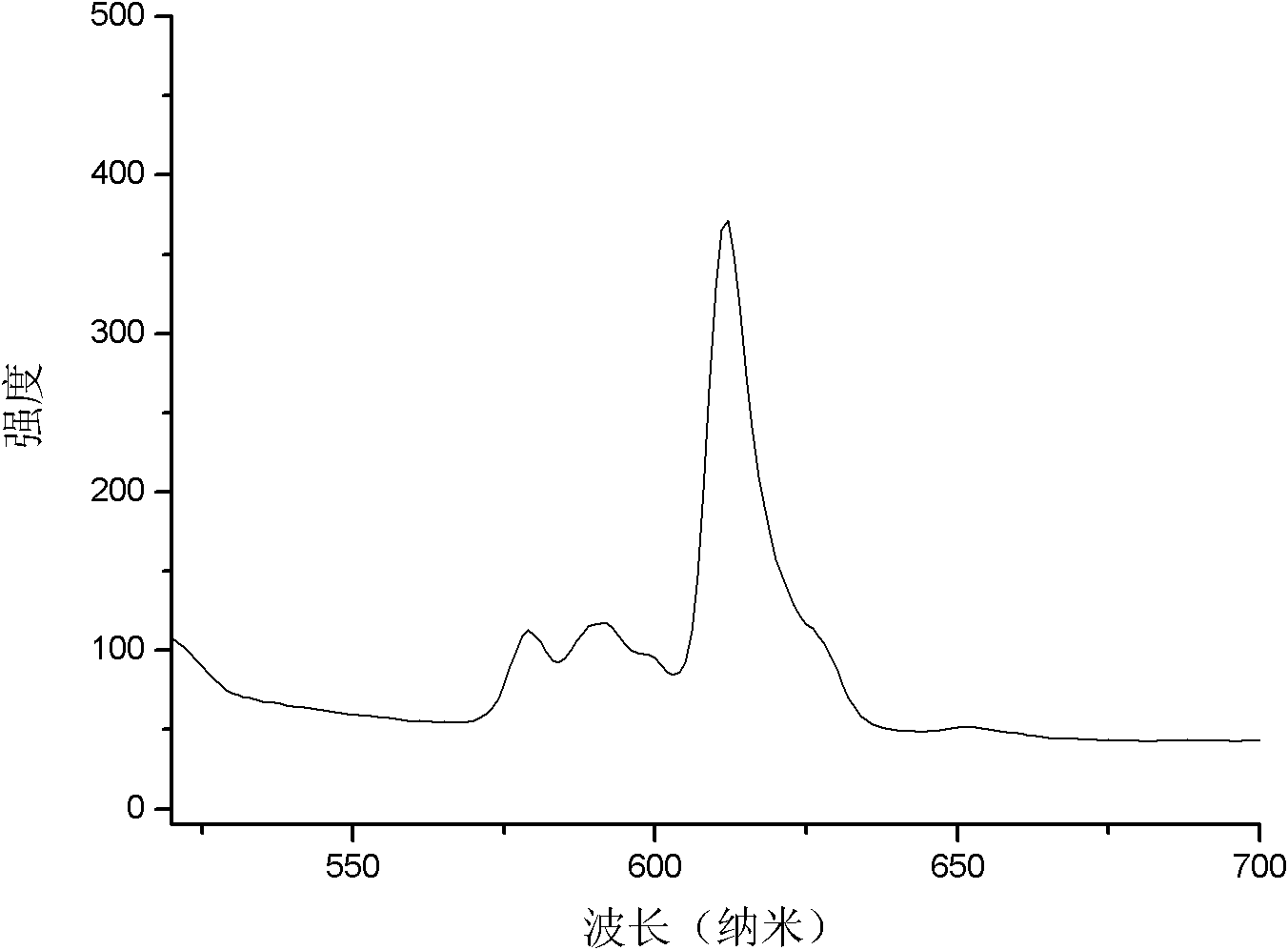
[0035] figure 2 It is the luminescence spectrum figure of the zinc oxide-based phosphor powder of the present...
Embodiment 2
[0037] Weigh 0.5179 g of chloroplatinic acid and dissolve in water to prepare 100 mL of chloroplatinic acid solution with a concentration of 0.01 mol / L.
[0038] Weigh 1.4280g of zinc nitrate and 0.0906g of terbium nitrate respectively, measure 1.44mL of diethanolamine and 2.5mL of chloroplatinic acid solution, and use ethylene glycol methyl ether as a solvent to prepare 50mL of zinc and terbium solutions containing 0.1mol / L. After stirring in a water bath at 70°C for 4 hours, a uniform precursor solution was obtained, and then the obtained precursor solution was aged in an oven at 60°C for 90 hours to obtain Zn 0.96 Tb 0.04 O: 0.005Pt colloid.
[0039] Dry the colloid obtained in the previous step in an oven at 200°C for 48 hours, and then place it in a corundum crucible and treat it at 1000°C for 3 hours in the presence of carbon powder to obtain Zn 0.96 Tb 0.04 O: 0.005Pt zinc oxide-based phosphor.
Embodiment 3
[0041] Weigh 1.6987 g of silver nitrate and dissolve it in water to prepare 1000 mL of silver nitrate solution with a concentration of 0.001 mol / L.
[0042] Take by weighing zinc chloride 1.3494g and erbium nitrate 0.0461g respectively, measure triethanolamine 2.64mL and silver nitrate solution 0.2mL, use ethylene glycol methyl ether as solvent to be mixed with the solution of 50mL containing 0.2mol / L zinc and erbium, After stirring in a water bath at 40°C for 8 hours, a uniform precursor solution was obtained, and then the obtained precursor solution was aged in an oven at 50°C for 56 hours to obtain Zn 0.99 Er 0.01 O: 0.00002Ag colloid.
[0043] Dry the colloid obtained in the previous step in an oven at 160°C for 70h, and then place it in a corundum crucible at 1200°C for 0.5h to obtain Zn 0.99 Er 0.01 O: 0.00002Ag zinc oxide-based phosphor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com