Synthetic method for preparing nano-cuprous oxide by nitrogen-doped cuprous oxide
A nano-cuprous oxide and cuprous oxide technology, applied in the direction of copper oxide/copper hydroxide, nanotechnology, etc., can solve the problem of high photogenerated electron-hole recombination rate, reduced photogenerated electron-hole recombination rate, and photocatalytic activity. It can achieve the effect of reducing the electron-hole recombination rate and improving the catalytic activity of visible light.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
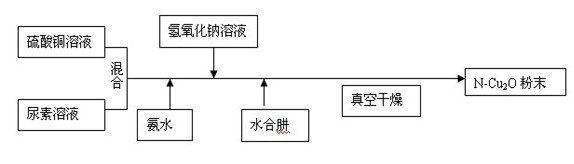
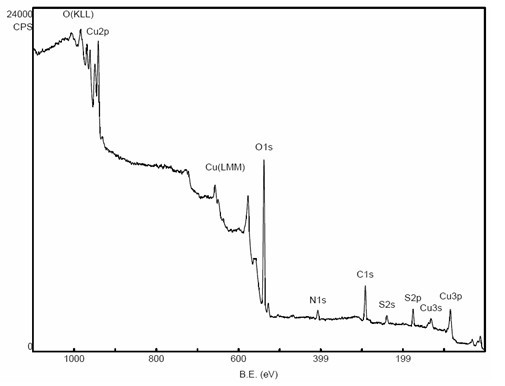
[0020] Example 1: 50ml of copper sulfate solution and 1ml of urea solution were stirred and mixed to obtain solution A; 15ml of ammonia solution with a concentration of 0.10 mol / L was added to solution A, and 5ml was added after stirring for 15 minutes, with a concentration of 1.5 mol / L After the solution changes from blue to light green, 2 ml of hydrazine hydrate with a concentration of 1.4 mol / L is added dropwise until the solution gradually changes from green to light green to obtain mixed solution B; after stirring the above solution B for 5h , the solution is suction filtered, and the filter cake is vacuum-dried to obtain nitrogen-doped nano-cuprous oxide.
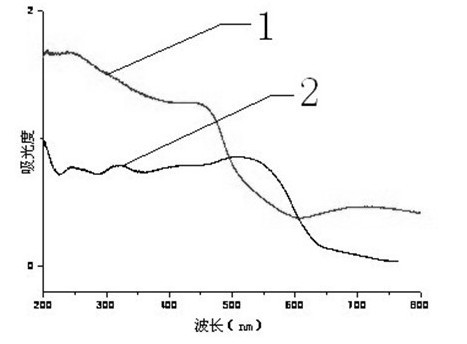
[0021] Add 0.05g of nitrogen-doped nano-cuprous oxide to 50ml of methylene blue solution (50mg / L), and place it in the dark for 24h to make it fully saturated with adsorption. Irradiated under the Lux fluorescent light source for 3.5 hours, took samples every half an hour, centrifuged, and took the supernatant to meas...
Embodiment 2
[0022] Embodiment 2: 50ml of copper sulfate solution and 1.5ml of urea solution are stirred and mixed to obtain A solution; 3ml of aqueous ammonia solution with a concentration of 0.15 mol / L is added in the A solution, and 10ml is added with a concentration of 0.8 mol / L after stirring for 30min. L sodium hydroxide solution, after the solution changes from blue to light green, add 7 ml of hydrazine hydrate with a concentration of 0.7 mol / L dropwise until the solution gradually changes from green to light green to obtain mixed solution B; stir the above solution B for 2h Then, the solution is suction filtered, and the filter cake is vacuum-dried to obtain nitrogen-doped nano-cuprous oxide.
[0023] Add 0.05g of nitrogen-doped nano-cuprous oxide to 50ml of methylene blue solution (50mg / L), and place it in the dark for 24h to make it fully saturated with adsorption. Irradiated under the Lux fluorescent light source for 3.5 hours, took samples every half an hour, centrifuged, and t...
Embodiment 3
[0024] Embodiment 3: 50ml of copper sulfate solution and 3.5ml of urea solution are stirred and mixed to obtain A solution; 10ml of ammonia solution with a concentration of 0.15mol / L is added to the A solution, and 7ml is added with a concentration of 1mol / L after stirring for 15min. After the solution changes from blue to light green, 3 ml of hydrazine hydrate with a concentration of 1 mol / L is added dropwise until the solution gradually changes from green to light green to obtain mixed solution B; after stirring the above solution B for 3 hours, The solution is suction filtered, and the filter cake is vacuum-dried to obtain nitrogen-doped nano-cuprous oxide.
[0025] Add 0.05g of nitrogen-doped nano-cuprous oxide to 50ml of methylene blue solution (50mg / L), and place it in the dark for 24h to make it fully saturated with adsorption. Irradiated under the Lux fluorescent light source for 3.5 hours, took samples every half an hour, centrifuged, and took the supernatant to measu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com