Preparation method of TiO2/BiOCl heterojunction visible light photocatalyst
A heterojunction, visible light technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of no research on BiOCl sheet/porous TiO, complicated preparation process, and low photocatalytic efficiency. The problem is to achieve the effect of high visible light catalytic activity, simple method and process, and simple equipment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 1.18g of Bi 2 o 3 , 0.30g of TiO 2, 4.68g of KCl and 3.67g of NaCl, put them in a ball mill jar and add 50ml of absolute ethanol, mix and ball mill for 3 hours, and dry at 80°C after ball milling to obtain powder A; put powder A into the muffle Calcined in the furnace at 800°C for 2h, cooled to room temperature with the furnace, fully washed the obtained product with deionized water to remove NaCl and KCl, and dried at 80°C to obtain the reaction precursor B; 0.12g of the reaction precursor B Put it into a beaker, add 30ml of deionized water, and add 0.5ml of hydrochloric acid with a mass fraction of 36.5%, and ultrasonically treat it for 0.5h to obtain a mixture C; heat the mixture C at 100°C for 2h, and dry to obtain TiO 2 / BiOCl heterojunction powder.
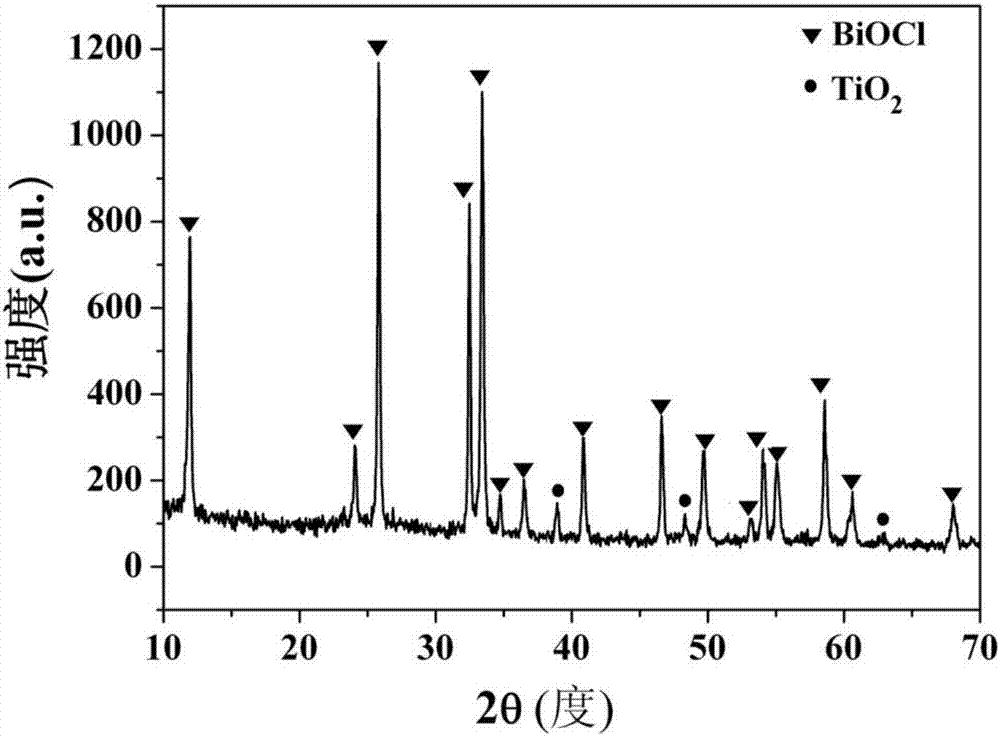
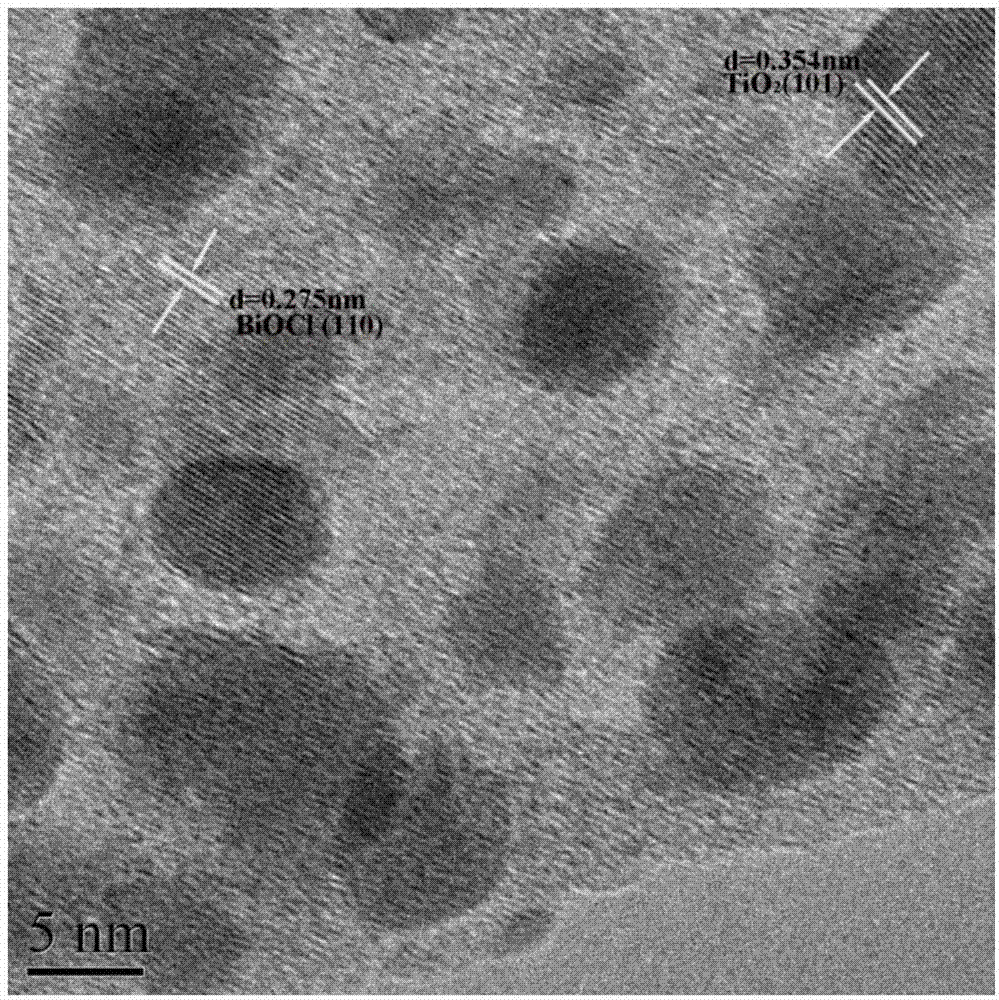
[0032] figure 1 TiO prepared for this example 2 / BiOCl heterojunction X-ray diffraction pattern, by figure 1 It can be seen that the product prepared in this embodiment is TiO 2 / BiOCl complex, and the Bi...
Embodiment 2
[0039] Weigh 2.36g of Bi 2 o 3 , 0.61g of TiO 2 , 9.36g of KCl and 7.31g of NaCl, put them in a ball mill jar and add 60ml of absolute ethanol, mix and ball mill for 5 hours, and dry at 100°C after ball milling to obtain powder A; put powder A into a muffle Calcined in the furnace at 1000°C for 1h, cooled to room temperature with the furnace, fully washed the obtained product with deionized water to remove NaCl and KCl, and dried at 80°C to obtain the reaction precursor B; 0.20g of the reaction precursor B Put it into a beaker, add 60ml of deionized water, and add 2ml of hydrochloric acid with a mass fraction of 36.5%, and sonicate for 1h to obtain a mixture C; heat the mixture C at 120°C for 3h, and dry to obtain TiO 2 / BiOCl heterojunction powder.
[0040] Using the above photocatalytic activity evaluation method, after visible light irradiation for 40min, the TiO prepared in this example 2 The degradation rate of rhodamine B by the / BiOCl heterojunction is about 91%, wh...
Embodiment 3
[0042] Weigh 1.18g of Bi 2 o 3 , 0.30g of TiO 2 , 7.45g of KCl and 5.84g of NaCl, put them in a ball mill jar and add 50ml of absolute ethanol, mix and ball mill for 4 hours, and dry at 100°C after ball milling to obtain powder A; put powder A into the muffle Calcined in the furnace at 900°C for 2h, cooled to room temperature with the furnace, the obtained product was fully washed with deionized water to remove NaCl and KCl, and dried at 80°C to obtain the reaction precursor B; 0.15g of the reaction precursor B Put it into a beaker, add 30ml of deionized water, and add 1.2ml of hydrochloric acid with a mass fraction of 36.5%, and sonicate for 0.5h to obtain a mixture C; heat the mixture C at 120°C for 2h, and dry to obtain TiO 2 / BiOCl heterojunction powder.
[0043] Using the above photocatalytic activity evaluation method, after visible light irradiation for 40min, the degradation rate of the TiO2 / BiOCl heterojunction prepared in this example to rhodamine B was about 89%,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com