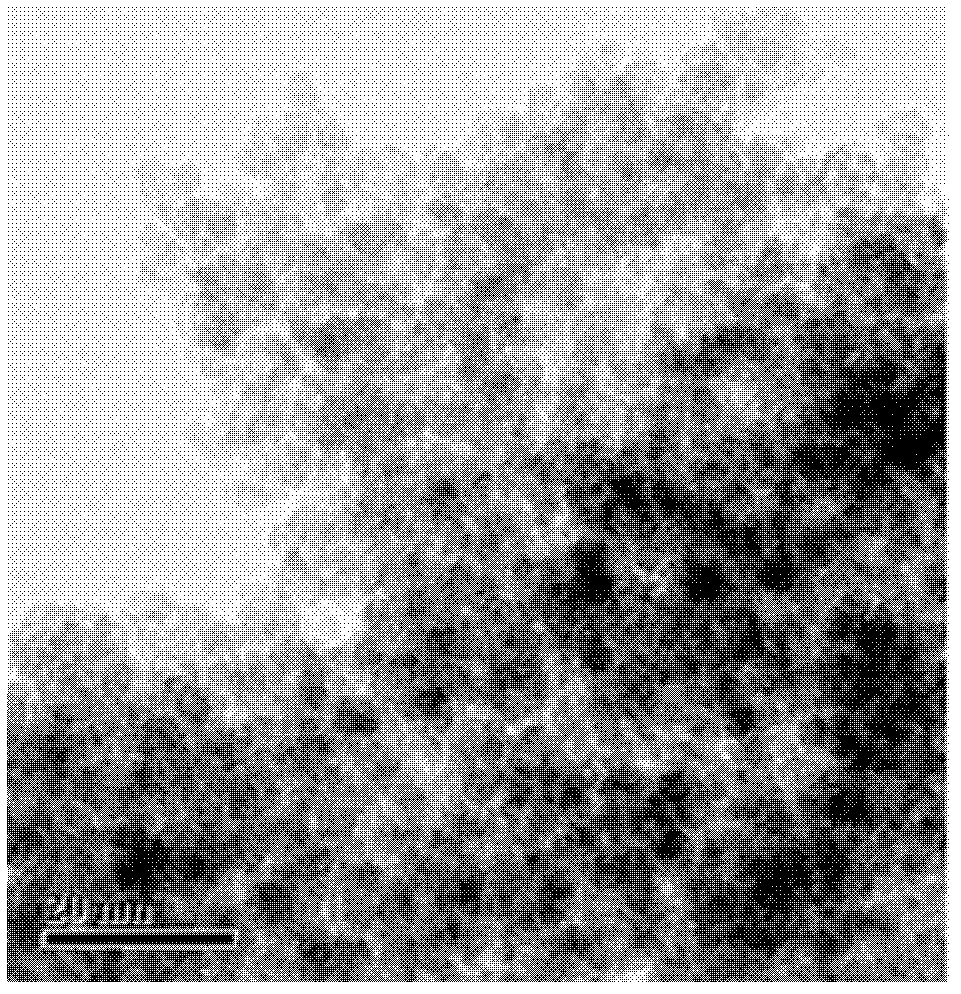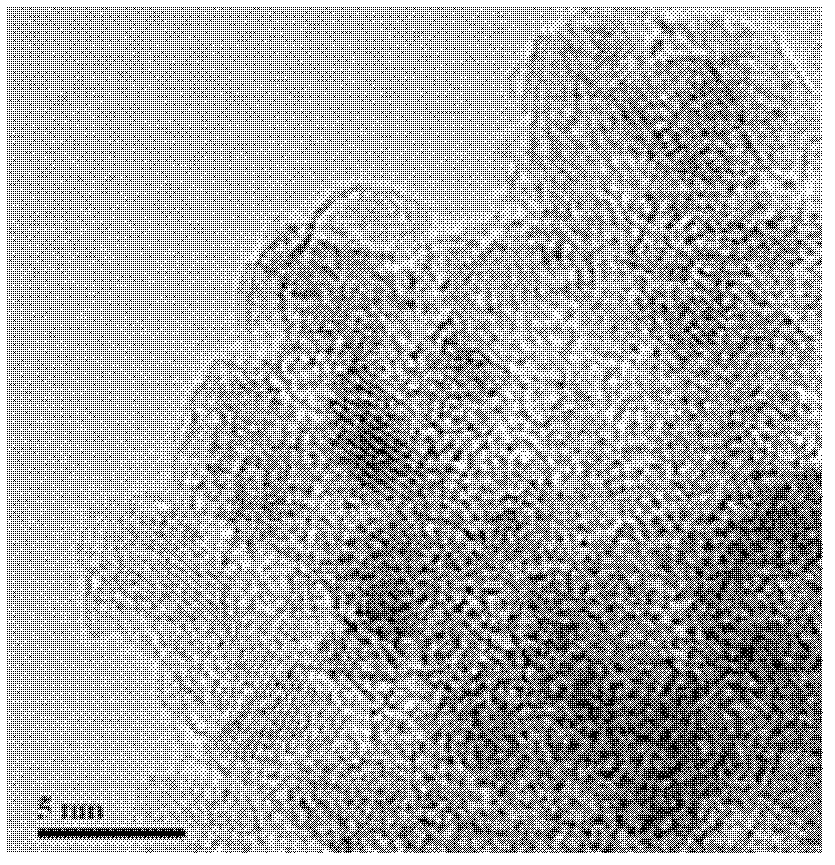Preparation method of magnetic Fe3O4 nanoparticles and its application in adsorption and separation of heavy metal ions
A heavy metal ion, nanoparticle technology, applied in nanotechnology, chemical instruments and methods, other chemical processes, etc., can solve the problems of expensive raw materials, high production costs, difficult control of reaction conditions, etc., and achieve simple synthesis methods and good adsorption. Features, low-cost and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Magnetic Fe 3 o 4 The preparation method of nanoparticles adopts a hydrothermal synthesis method, using water as a solvent, and the specific steps are:
[0026] 1) Add 0.27gFeCl 3 ·6H 2 O was dissolved in 40.5mL of water to obtain an aqueous ferric chloride solution, and then 0.162g of ascorbic acid was added to obtain a solution;
[0027] 2) Add 1 mL of hydrazine hydrate to the solution obtained in step 1), then transfer to a stainless steel reactor and react at 140°C for 24 hours. After the reaction, centrifuge at 4500 rpm for 30 minutes to obtain a precipitate. The obtained The precipitate was washed 3 times alternately with ethanol and water, and dried in a vacuum oven at 40 °C for 8 h to obtain magnetic Fe 3 o 4 Nanoparticles, which are spherical, have a particle size of 3±1nm, and their transmission electron microscope pictures are as follows: figure 1 As shown, the high-resolution transmission electron microscope picture is shown as figure 2 shown.
[00...
Embodiment 2
[0031] Magnetic Fe 3 o 4 The preparation method of nanoparticles adopts a hydrothermal synthesis method, using water as a solvent, and the specific steps are:
[0032] 1) Add 0.27gFeCl 3 ·6H 2 O was dissolved in 40mL of water to obtain an aqueous ferric chloride solution, and then 0.405g of ascorbic acid was added to obtain a solution;
[0033] 2) Add 1.5mL of hydrazine hydrate to the solution obtained in step 1), then transfer to a stainless steel reactor and react at 160°C for 12 hours. After the reaction, centrifuge at a speed of 4500 rpm for 30 minutes to obtain a precipitate, which will be obtained The precipitate was alternately washed 3 times with ethanol and water, and dried in a vacuum oven at 50 °C for 6 h to obtain magnetic Fe 3 o 4 Nanoparticles, for the cubic phase of ferroferric oxide, its X-ray diffraction pattern is as follows Image 6 shown. Test instrument model: XRD-6000; test conditions: scan range 10-70°, scan rate 0.02 / s -1 .
[0034] 7 mg of mag...
Embodiment 3
[0037] Magnetic Fe 3 o 4 The preparation method of nanoparticles adopts a hydrothermal synthesis method, using water as a solvent, and the specific steps are:
[0038] 1) Add 0.27gFeCl 3 ·6H 2 O was dissolved in 40 mL of water to obtain an aqueous solution of ferric chloride, and then 0.54 g of ascorbic acid was added to obtain a solution;
[0039] 2) Add 2.6mL of hydrazine hydrate to the solution obtained in step 1), then transfer to a stainless steel reactor and react at 200°C for 6 hours. After the reaction, centrifuge at 4500 rpm for 30 minutes to obtain a precipitate, which will be obtained The precipitate was alternately washed 3 times with ethanol and water, and dried in a vacuum oven at 60 °C for 4 h to obtain magnetic Fe 3 o 4 Nanoparticles, whose hysteresis loop at room temperature is as Figure 8 As shown, it can be seen from the figure that the obtained magnetic Fe 3 o 4 Nanoparticles exhibit superparamagnetism with a saturation magnetic susceptibility clos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturation susceptibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com