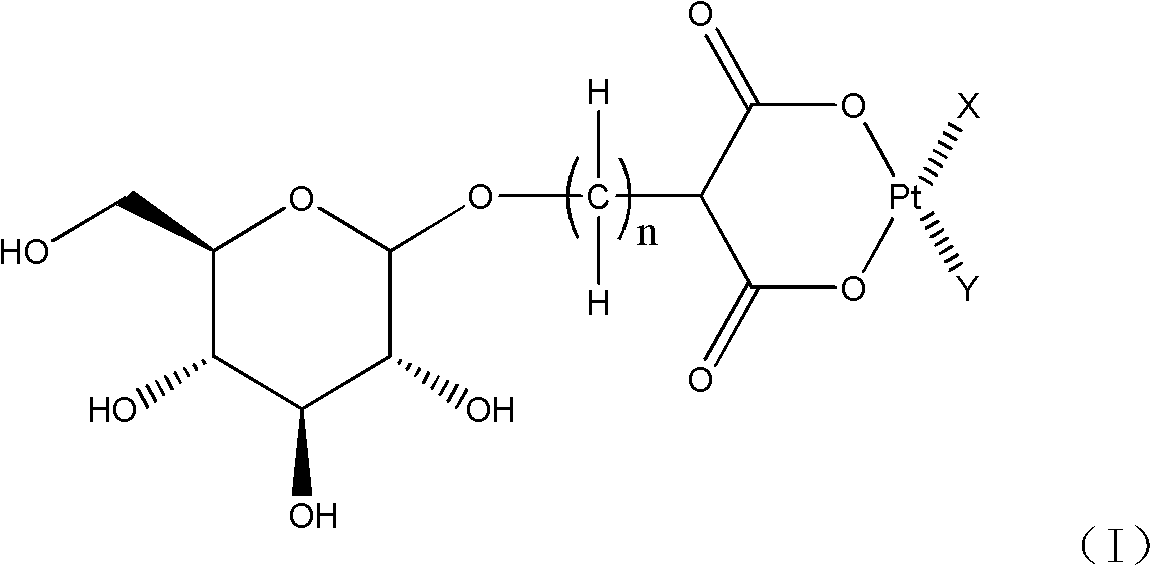
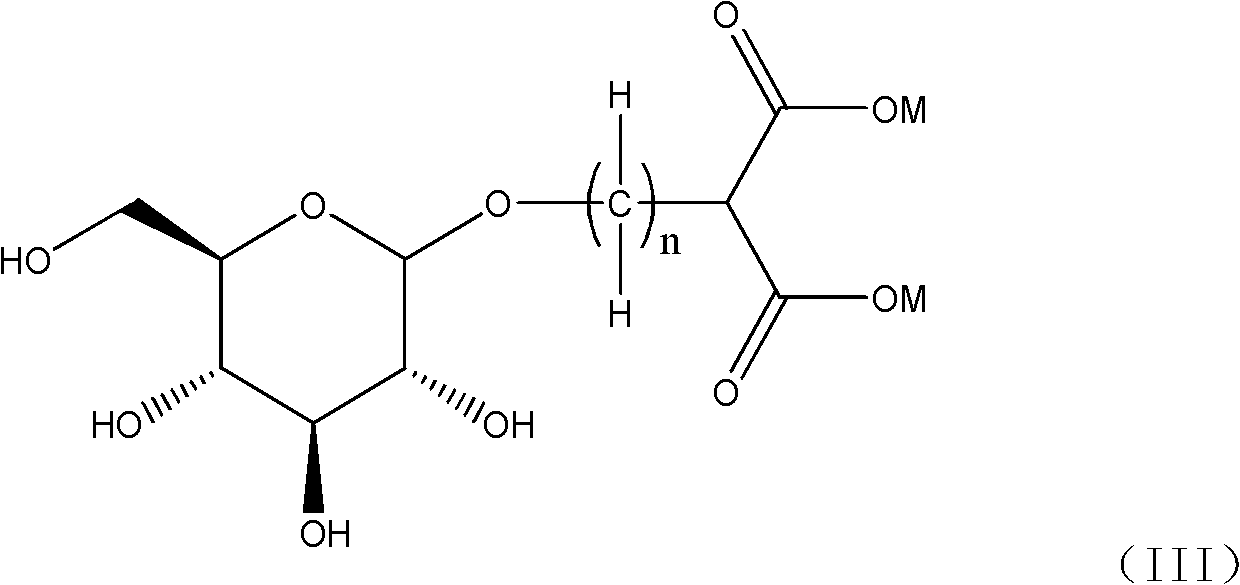
Glucose-containing platinum complex for treating tumors and preparation method thereof
A technology of tumor targeting and platinum complexes, which is applied in the preparation of sugar derivatives, medical preparations containing active ingredients, sugar derivatives, etc., can solve the problem of increased toxicity and side effects, difficult renal elimination, and no fundamental solution Platinum resistance and other problems, to achieve the effect of high water solubility and clinical application of tumor targets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The specific preparation can be accomplished using the following methods and reaction formulas.
[0051] Method A:
[0052]
[0053] Method B:
[0054]
[0055] In method A, when M is a hydrogen atom in formula (III), the reaction can be carried out by using an appropriate inorganic base, such as sodium hydroxide, potassium hydroxide, sodium carbonate, sodium bicarbonate, potassium carbonate, lithium hydroxide and hydrogen cesium oxide etc. to adjust the pH of the reaction aqueous solution and maintain between 7-9 to complete the preparation of the compound shown in formula (I); when M is a metal atom, for example: sodium atom, potassium atom, lithium atom, barium atom or cesium atoms, the reaction can proceed smoothly in an aqueous solution, and if necessary, use a small amount of the aqueous solution of the above-mentioned inorganic base to maintain the pH of the reaction solution between 7-9 to complete the synthesis of the complex shown in formula (I).
[00...
Embodiment 1
[0077] (1) Preparation of 1-O-D-glucoside-2-bromo-ethane:
[0078]
[0079] 1) Add glucose (2.7g) to 2-bromoethanol (10ml) at room temperature, replace the air in the flask with nitrogen after cooling to 0°C, and slowly add boron trifluoride ether solution dropwise under nitrogen protection (98%, 1 ml).
[0080] 2) Stir the reaction solution at 0°C for 15 minutes, then slowly warm up to room temperature and stir for 30 minutes, then heat the reaction solution to 80°C, and react at 80°C for 5 hours.
[0081] After the reaction was completed, the solvent was removed by rotary evaporation, and the reaction product was simply purified by silica gel column chromatography (dichloromethane:methanol, 6:1) to obtain 2.3 g of crude product.
[0082] Mass Spectrum: MS, m / z: 287.23 [M+H] +
[0083] (2) Preparation of 1-O-(2,3,4,6-tetraacetyl-D-glucoside)-2-bromo-ethane:
[0084]
[0085] At room temperature, 2.3 g of the product 1-O-D-glucoside-2-bromo-ethane obtained in the pre...
Embodiment 2
[0138] (1) Preparation of 1-O-D-glucoside-3-bromo-propane:
[0139]
[0140] Glucose (1.8g) was added to 3-bromopropanol (8mL) at room temperature, cooled to 0°C, and the air in the flask was replaced with nitrogen, and the ether solution of boron trifluoride ( 98%, 0.7 mL). The reaction solution was stirred at 0°C for 15 minutes, raised to room temperature and stirred for 30 minutes, then heated to 80°C, and reacted at 80°C for 5 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and 2 g of crude product was obtained after simple purification by silica gel column chromatography (dichloromethane:methanol, volume ratio 6:1).
[0141] Mass spectrum: MS, m / z: 301.23 [M+H] +
[0142] (2) Preparation of 1-O-(2,3,4,6-tetraacetyl-D-glucoside)-3-bromo-propane:
[0143]
[0144] At room temperature, 2 g of the crude product of 1-O-D-glucoside-3-bromo-propane obtained in the previous step was dissolved in pyridine and acetic anhydride (6ml:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com