Method for preparing 5-nitro vanillin
A technology of nitrovanillin and vanillin, applied in the field of preparation of 5-nitrovanillin, which can solve the problems of complex product separation process, large amount of waste acid waste water, increased side reactions, etc., and shorten the reaction time , low production cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
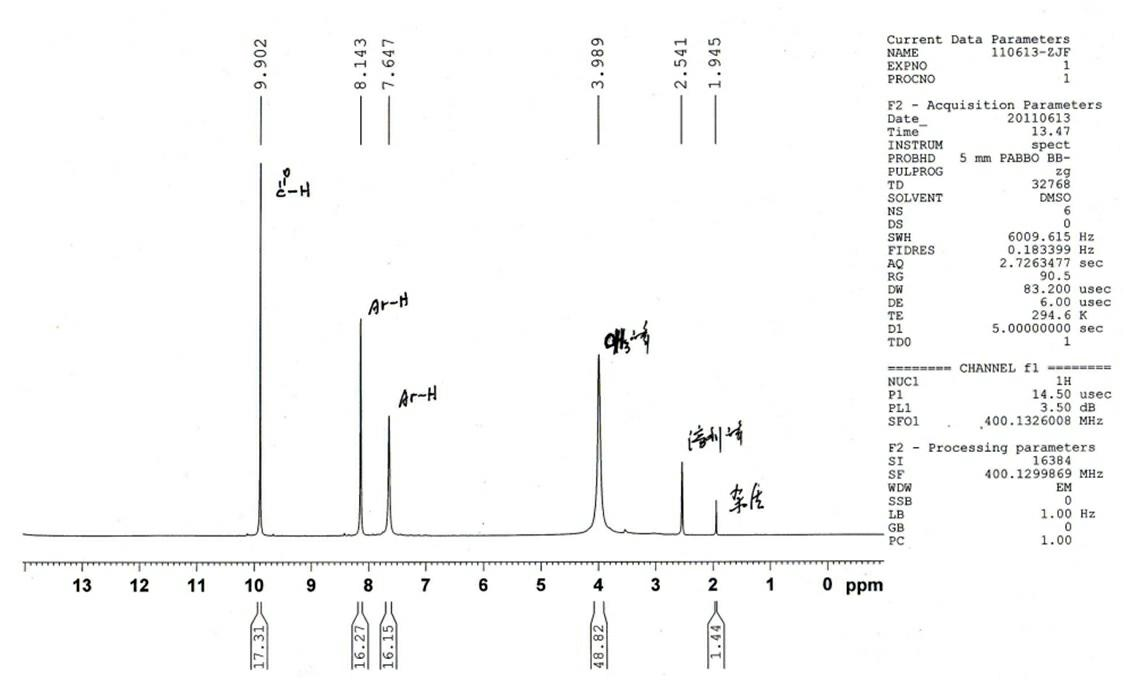
[0013] In a 25mL Erlenmeyer flask, add 0.152g (1mmol) vanillin, 2mL volume fraction 90% acetic acid, 0.50g (1.25mmol) polyethylene glycol-400, slowly drop in 0.09g (0.58mmol) under stirring condition Aqueous solution of ammonium cerium nitrate, reacted at 20°C for 2.5 hours, tracked by TLC, developer: ethyl acetate: petroleum ether = 1: 1, stop stirring when the reactant is completely converted into product; pour the reactant into an appropriate amount of ice water , a yellow solid was precipitated, suction filtered, and the solid was washed 2-3 times with distilled water to obtain the crude product of 5-nitrovanillin; weighing: 0.14g, yield: 71%; the product was determined by melting point and infrared The spectrum and proton nuclear magnetic resonance spectrum analysis are consistent with the literature reports; the measured melting point is: m.p: 177.2~178.6 ℃;
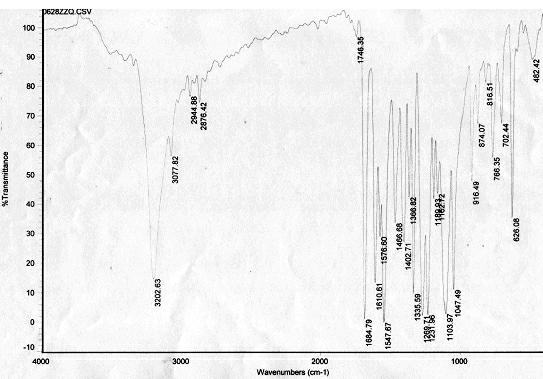
[0014] IR: ν max (KBr pellets, cm -1 ), 3203, 3077, 2945, 2876, 1685, 1611, 1548, 1402, 1335, 1269, 1231, 1103...
Embodiment 2
[0016] In a 100mL Erlenmeyer flask, add 0.76g (5mmol) vanillin, 10mL volume fraction 90% acetic acid, 2.5g (6.25mmol) polyethylene glycol-400, slowly drop in 0.76g (4.9mmol) under stirring condition Aqueous solution of ammonium cerium nitrate; react at 40°C for 1.5 hours, tracked by TLC, developer: ethyl acetate: petroleum ether = 1:1, stop stirring when the reactant is completely converted into product; pour the reactant into an appropriate amount of ice water , a yellow solid was precipitated, suction filtered, and the solid was washed 2-3 times with distilled water to obtain the crude product of 5-nitrovanillin; weighing: 0.68g, yield: 69%; the measured melting point was: m.p : 177.5~178.6 ℃.
Embodiment 3
[0018] Add 15.2g (100mmol) vanillin, 200mL volume fraction 90% acetic acid, 50.0 g (125 mmol) polyethylene glycol-400 into a 1000mL round-bottomed flask, slowly drop into 24.32g (158 mmol) ) aqueous solution of ammonium cerium nitrate, reacted at 60°C for 1.0 hour, tracked by TLC, developer: ethyl acetate: petroleum ether = 1: 1, stop stirring when the reactant is completely converted into product; pour the reactant into an appropriate amount of ice In the water, a yellow solid was precipitated, filtered by suction, and washed with distilled water for 2-3 times to obtain a crude product of 5-nitrovanillin; weighing: 13.8 g, yield: 70%. The measured melting point is: m.p: 177.5~178.5 ℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com