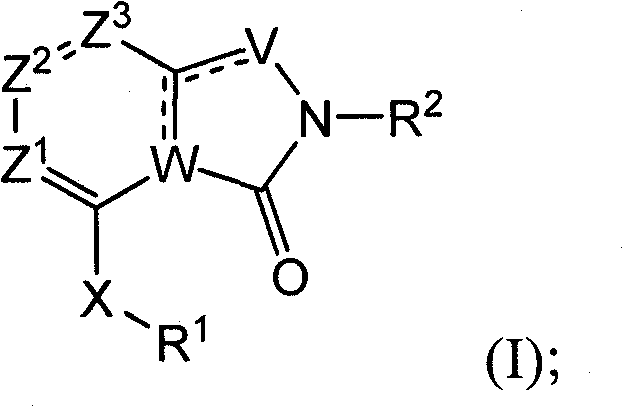
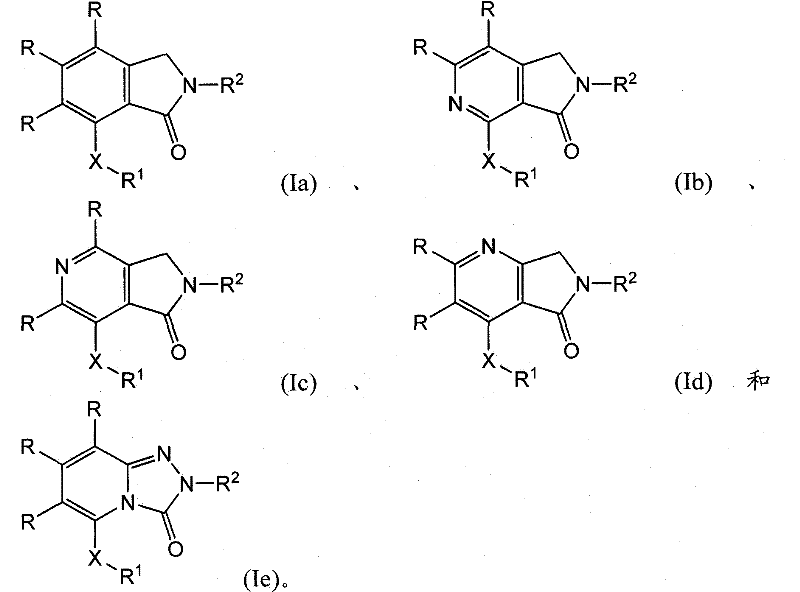
Isoindolinone and related analogs as sirtuin modulators
A compound, alkyl technology, applied in the field of isoindolinone and related analogues as sirtuin regulators, which can solve the problem of reducing and extending the lifespan of wild-type cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0491] Example 1 Preparation of N-(3-oxo-2-(3-(trifluoromethyl)phenyl)isoindoline-4-yl)pyrazine-2-carboxamide (compound 100):
[0492] Step 1) Synthesis of 2-methyl-6-nitrobenzoic acid methyl ester (2):
[0493]
[0494] 2-Methyl-6-nitrobenzoic acid (1; 10 g, 0.0552 mol) was dissolved in 200 mL of methyl ethyl ketone together with methyl iodide (17.2 mL, 0.276 mol) and anhydrous potassium carbonate (38.1 g, 0.276 mol) . The reaction mixture was stirred at reflux for 18 hours. It was then cooled to room temperature and filtered. The filtrate was diluted with EtOAc (300 mL), washed with water (2 x 50 mL), brine, dried (Na 2 SO 4 ), and concentrated under reduced pressure to obtain methyl 2-methyl-6-nitrobenzoate 2 (10.80g).
[0495] Step 2) Synthesis of 2-(bromomethyl)-6-nitrobenzoic acid methyl ester (3):
[0496]
[0497]Methyl 2-methyl-6-nitrobenzoate (2; 10.8g, 0.0552mol) was mixed with N-bromosuccinimide (9.82g, 0.0552mol) and 100mg of azo-bis(isobutyronitrile ...
Embodiment 2
[0507] Example 2: Preparation of additional N-(3-oxo-2-(optionally substituted phenyl)isoindolin-4-yl)carboxamides according to the invention:
[0508] Synthesis of 7-nitro-2-(3-(trifluoromethoxy)phenyl)isoindolin-1-one (6):
[0509]
[0510] Methyl 2-(bromomethyl)-6-nitrobenzoate (from Example 1, step 2) (3; 0.86 g, 3.14 mmol), 3-(trifluoromethoxy ) aniline (21; 0.56g, 3.14mmol) and HOAc (0.3mL) in di The mixture in alkanes (3 mL) was stirred for 20 minutes. The mixture was adjusted to pH = 7 and extracted with ethyl acetate (3 x 25 mL). The combined organic layers were washed with Na 2 SO 4 Drying, concentration in vacuo, and purification by silica gel chromatography afforded 7-nitro-2-(3-(trifluoromethoxy)phenyl)isoindolin-1-one 6 (0.67 g, 1.98 mmol, 63 %). MS(ESI)C 15 h 9 f 3 N 2 o 4 Calculated: 338; Measured: 339 [M+H]. 7-nitro-2-(3-(trifluoromethoxy)phenyl)isoindolin-1-one 6 was converted into the corresponding amine, and then according to the steps 3-4 m...
Embodiment 3
[0512] Example 3: N-(3-oxo-2-(3-(piperidin-4-yloxy)phenyl)isoindoline-4-yl)-2-(pyridin-3-yl)ethyl Preparation of Amide (Compound 102):
[0513] Step 1) Synthesis of 4-(3-nitrophenoxy)piperidine-1-carboxylic acid tert-butyl ester (9):
[0514]
[0515] 4-Hydroxypiperidine-1-carboxylic acid tert-butyl ester (8; 6.27 g, 47.44 mmol), 3-nitrophenol (7; 6.0 g, 43.13 mmol) and triphenylphosphine ( To a solution of 12.44 g, 47.44 mmol) in anhydrous THF (40 mL) was added diethyl azodicarboxylate (8.26 g, 47.44 mmol) dropwise. The mixture was then warmed to room temperature and stirred overnight. The reaction mixture was concentrated and then purified by column chromatography (EtOAc:petroleum ether=1:6) to give tert-butyl 4-(3-nitrophenoxy)piperidine-1-carboxylate 9 as an oil (8.2 g) . MS(ESI)C 16 h 22 N 2 o 5 Calculated: 322; Measured: 323 [M+H].
[0516] Step 2) Synthesis of 4-(3-aminophenoxy)piperidine-1-carboxylic acid tert-butyl ester (10):
[0517]
[0518] tert-Bu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com