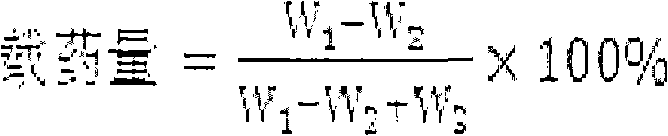Jasminoidin or gardenia total iridoid glycoside liposome preparation
A technology for total iridoid glycosides and liposome preparations, applied in the field of pharmaceutical preparations, can solve problems such as poor ability to penetrate mucous membranes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
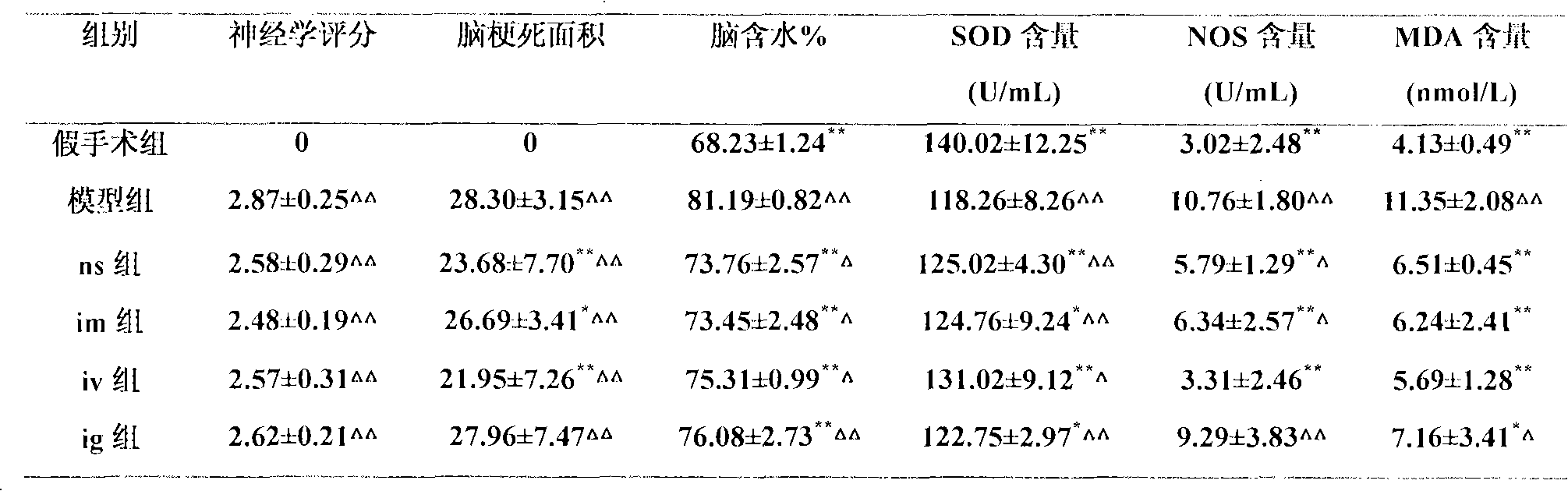
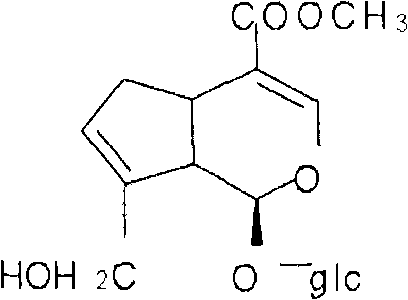
[0077] The preparation of embodiment 1 gardenia total iridoid glycosides
[0078]Take 1 kg of gardenia coarse powder, add 6 times of water to decoct 3 times, each time for 30 minutes, filter, combine the decoction, concentrate to 1000 ml of thick paste (each 1 ml is equivalent to 1 g of medicinal materials), add 730 ml of 95% ethanol to contain Alcohol content 40%, stand for 24 hours, take the supernatant, recycle ethanol, concentrate to 500ml of thick cream with no alcohol smell (each 1ml is equivalent to 2g of medicinal materials), add 2700ml of 95% ethanol to alcohol content of 80%, stand for 24 hours, Filter, the filtrate is concentrated to a relative density of 1.05 to 1.10 (60° C.), and dried to obtain 130 g of dry cream. The content of total iridoid glycosides of geniposide is determined to be 55% by ultraviolet spectrophotometry, and the content of total iridoid glycosides of geniposide is determined by high-performance liquid phase. The glycoside content is 27.4%.
Embodiment 2
[0079] Embodiment 2 reverse evaporation method prepares geniposide liposome
[0080] Dissolve 50 mg of geniposide and 9 mg of sodium chloride in 10 ml of PBS (PH=7.0) buffer solution, and dissolve 160 mg of egg yolk phospholipid (EPC) and 40 mg of cholesterol in 30 ml of organic phase (chloroform:isopropanol=14:1 ), the two were mixed, and the water bath was sonicated for 8 minutes until a stable water / oil (W / O) emulsion was formed (the temperature of the water bath was controlled at 20 ° C), and then the organic solvent was evaporated in a rotary evaporator under reduced pressure, and the colloidal state was dropped Add 1-2ml of PBS (PH=7.0) buffer solution, hydrate, and continue to evaporate under reduced pressure for a short time to make a white liposome suspension. Add preservatives and fill in nasal cavity drug delivery devices.
Embodiment 3
[0081] Example 3 Preparation of total iridoid glycoside liposomes of Gardenia jasminoides by reverse evaporation method
[0082] Take total iridoid glycosides of gardenia jasmine glycosides 50mg and sodium chloride 9mg prepared in Example 1 and dissolve them in 10ml of PBS (PH=7.0) buffer solution, and take soybean lecithin (SPC) 100mg and cholesterol 50mg and dissolve them in 30ml of organic phase (Chloroform: isopropanol = 14:1), mix the two, and ultrasonicate in a water bath for 8 minutes to obtain a white emulsion. Evaporate the organic solvent in a water bath on a rotary evaporator (to make it into a gel), and add a small amount of PBS (PH=7.0) to buffer Solution, hydration, and continue to evaporate under reduced pressure for a short time to make a liposomal suspension. Add preservatives and fill in nasal cavity drug delivery devices.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com