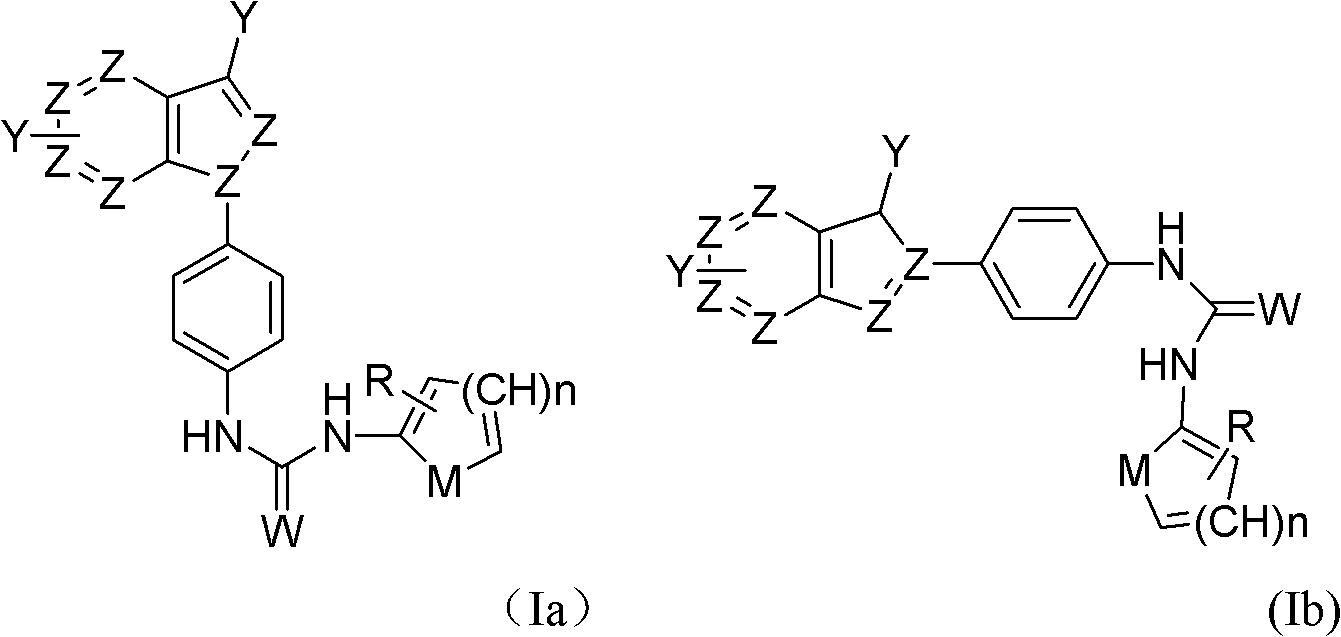
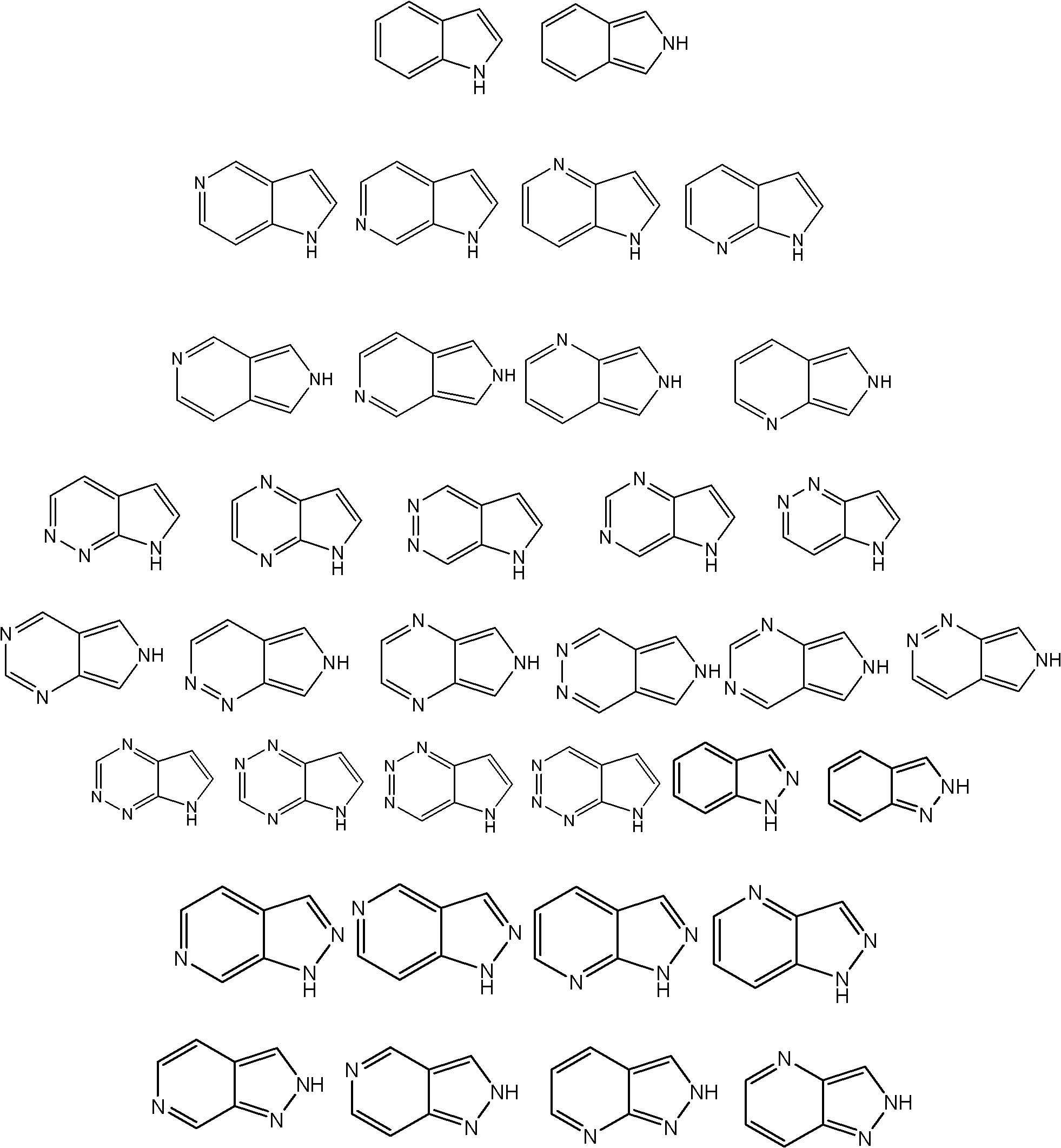
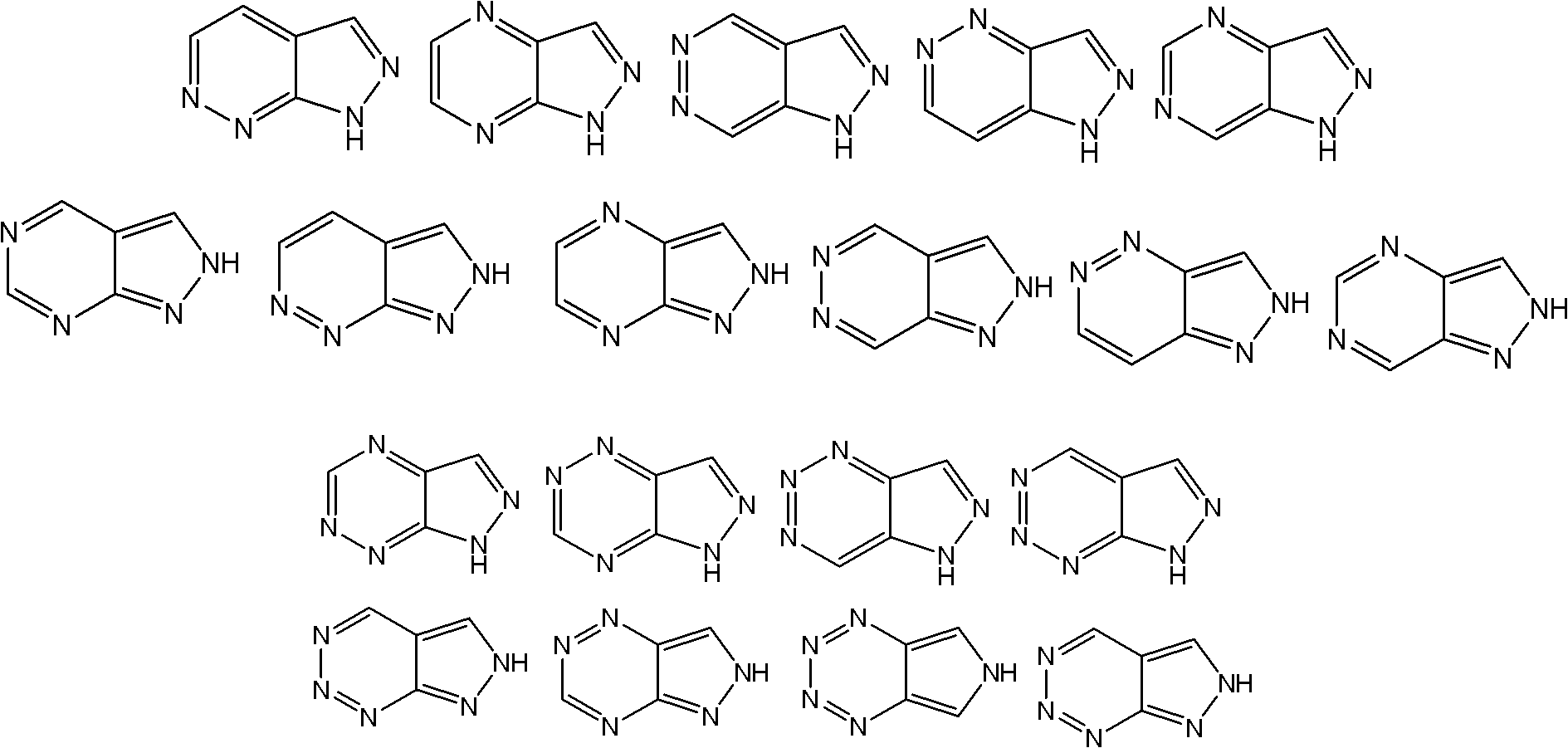
Application of indazole bi-aryl urea compound serving as protein kinase inhibitor
A technology for indazole biaryl urea and protein kinase, which is applied in the field of indazole biaryl urea compounds as protein kinase inhibitors, and can solve problems such as other uses that have not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, kinase test
[0041] experiment method:
[0042] The KINOMEscan screening platform is currently the most comprehensive, using a novel, proprietary active-site-directed competitive binding assay to quantify the interaction of test compounds with 450 human kinases and diseases associated with mutant variants. A system for high-throughput screening of compounds targeting human kinases. The test is carried out by combining with three compounds: DNA-labeled kinase, immobilized ligand, and test compound. The ability of the test compound to compete with the immobilized ligand was detected by quantitative PCR using DNA labeling.
[0043] Most of the experiments were performed on kinase-tagged T7 phage strains grown in E. coli host cells derived from the BL21 strain. E. coli was grown to logarithmic phase, infected with T7 phage, and incubated at 32° C. with shaking until the E. coli lysed. The lysate was centrifuged and filtered to remove cell debris. The rem...
Embodiment 2
[0059] Embodiment 2, pharmacokinetic experiment
[0060] 1. Experimental materials
[0061] 1. Reagents, medicines and consumables
[0062] Compound 1, compound 1 mesylate (1S), compound 2, compound 2 mesylate (2S), acetonitrile, ethyl acetate, methanol, column Hypersil ODS (4.6mm×200mm, 5mm), centrifugal tube, EP tube, pipette tip, rubber gloves, 1mL syringe, etc.
[0063] 2. Main instruments
[0064] Agilent 1200 high performance liquid chromatography (Agilent Technology Co., Ltd. of the United States); SHZ-88 water bath constant temperature oscillator (Jintan Cultural Instrument Factory Co., Ltd.); KQ3200DB ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.); UV-2102PCS Type UV Spectrophotometer (Shanghai Longnic Instrument Co., Ltd.); TGL-16 Desktop Centrifuge (Shanghai Anting Scientific Instrument Factory); XW-80A Vortex Mixer (Shanghai Jingke Industrial Co., Ltd.); Swiss Mettler- Toledo PL203 electronic balance (Switzerland).
[0065] 3. Experimental animal...
Embodiment 3
[0101] Embodiment 3, to S180 mouse sarcoma growth inhibition experiment
[0102] 1. Experimental materials
[0103] 1. Test drugs: Compounds 1 and 2;
[0104] 2. Experimental animals: Kunming mice weighing 19-22 g were purchased from the Experimental Center of Shandong University.
[0105] 3. Mouse S180 sarcoma: ascites was preserved for passage, purchased from Institute of Materia Medica, Shandong Academy of Medical Sciences.
[0106] 4. Other reagents and experimental equipment: physiological saline, ultra-clean workbench, laminar flow frame for large / mouse, surgical instruments, syringes, intragastric needles, autoclaves, etc.
[0107] 2. Experimental method
[0108] 1. Tumor cell inoculation method: Take the mice with S180 ascites for 5-7 days, extract the tumor cell fluid under aseptic conditions, count and dilute, and inoculate the right axillary subcutaneous of the mice, and the number of cells inoculated in each mouse is 2.5 ×10 6 / 0.2ml / piece. On the day after i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com