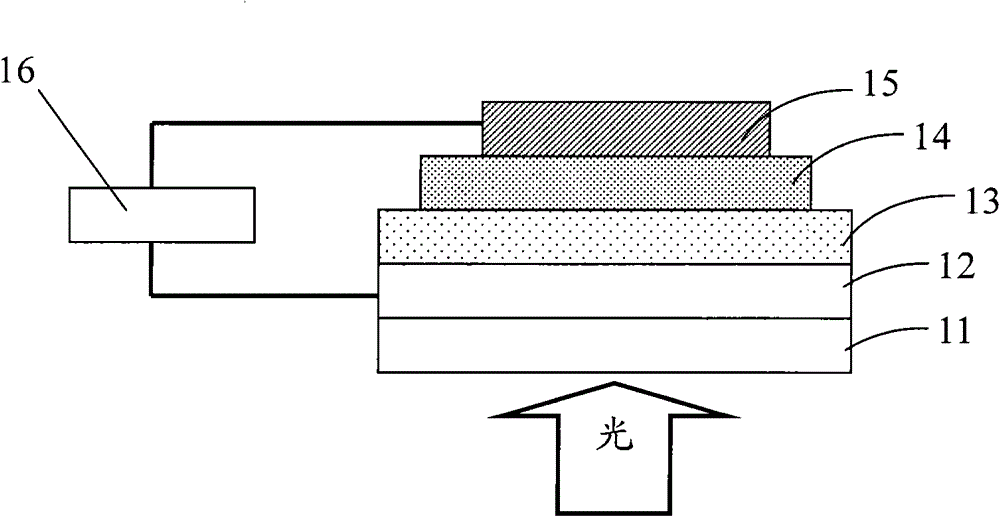
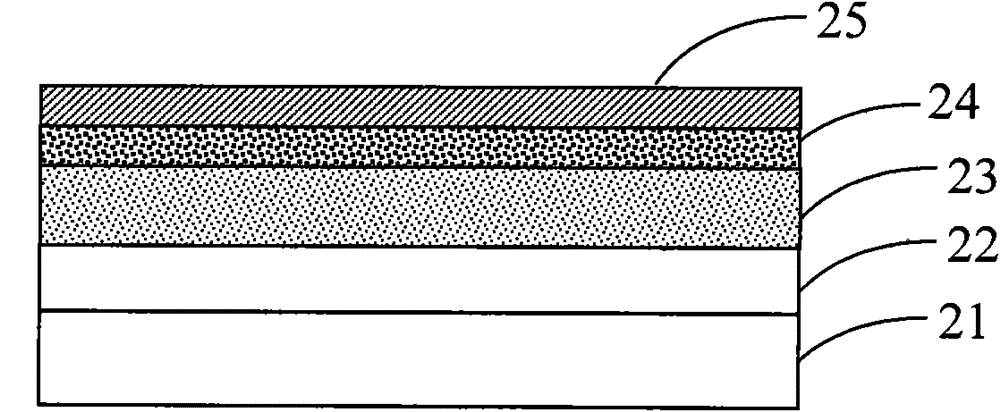
Fluorene-based copolymer containing thiophene and thiophene pyrroledione units, its preparation method and application
A technology of diketothiophene pyrrole and copolymer, which can be used in electrical components, structure of active regions, semiconductor/solid-state device manufacturing, etc., and can solve problems such as low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
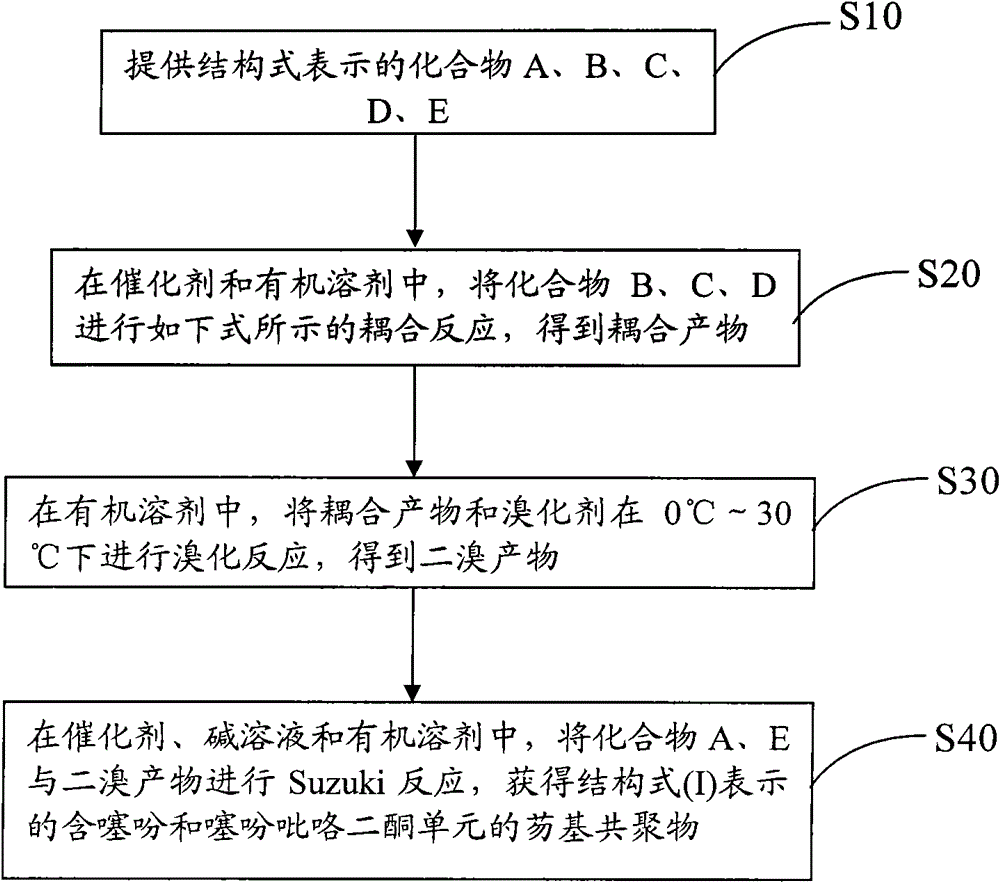
[0034] see figure 1 , the preparation method of the above-mentioned fluorenyl copolymer containing thiophene and thiophene pyrrole diketone unit comprises the following steps:
[0035] S10: provide compounds A, B, C, D, E represented by the following structural formula,
[0036]
[0037] Where: R 1 , R 2 , R 3 from C 1 -C 20 the alkyl group; R 4 , R 5 , R 6 , R 7 , R 8 , R 9 Choose from H or C 1 -C 20 The alkyl group; m is an integer of 1-5;
[0038] S20: In a catalyst and an organic solvent, compound B, C, and D are subjected to the coupling reaction shown in the following formula to obtain a coupling product;
[0039]
[0040] S30: In an organic solvent, the coupling product and the brominating agent are subjected to a bromination substitution reaction to obtain a dibrominated product;
[0041] S40: In catalyst, alkaline solution and organic solvent, carry out Suzuki reaction with compound A, compound E and dibromo product, obtain the fluorenyl copolym...
Embodiment 1
[0070] The fluorene-based copolymer (I 1 ), R 1 , R 2 , R 3 Both are methyl, R 4 , R 5 , R 6 , R 7 Both are H, R 8 , R 9 All are methyl, x=1 / 3, y=2 / 3, m=1, n=10, and its structural formula is as follows:
[0071]
[0072] The copolymer represented by the above structural formula (I 1 ), R 4 , R 5 , R 6 , R 7 Both are H, indicating that compounds C and D have the same structure, so only one kind of raw material needs to be provided, and the reaction yield is improved.
[0073] The copolymer of the present embodiment (I 1 ) preparation comprises the following specific steps:
[0074] 1. The preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylsilfluorene, compound A A concrete example, its structural formula is as follows:
[0075]
[0076] The specific preparation process is as follows: Add 20.00mL (1.00mol / L) n-butyllithium solution to 3.52g of 2,7-dibromo-9,9-dimethylsilane at -100°C under nitrogen gas In the reactor of fluorene a...
Embodiment 2
[0095] The fluorene-based copolymer (I 2 ), R 1 , R 2 , R 3 Both are C 8 h 17 , R 4 , R 5 , R 6 , R 7 Both are H, R 8 , R 9 Both are C 8 h 17 , x=1 / 2, y=1 / 2, m=1, n=39, its structural formula is as follows:
[0096]
[0097] Similar to Example 1, the copolymer represented by the above structural formula (I 2 ), R 4 , R 5 , R 6 , R 7 Both are H, indicating that compounds C and D have the same structure, so only one kind of raw material needs to be provided, and the reaction yield is improved. Moreover, R 1 , R 2 , R 3 and R 8 , R 9 Both are C 8 h 17 , which is beneficial to improve the copolymer (I 2 ) molecular weight, which is conducive to processing and forming a film, such as facilitating the use of spin coating technology to form a film.
[0098] The copolymer of the present embodiment (I 2 ) preparation comprises the following specific steps:
[0099] One, the preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluorene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com