Pharmaceutical composition for lowering blood lipid
The technology of a composition and a lipid-lowering drug, applied in the field of medicine, can solve the problems of unsatisfactory treatment effect and the like, and achieve the effect of significant curative effect and lowering cholesterol.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0014] Specific Example 1 Ezetimibe / niacin composition
[0015] In this example, niacin and corresponding pharmaceutical excipients were used to prepare tablet cores, and the corresponding OPadry II clear suspension was sprayed on the niacin tablet cores to prepare a separation layer between niacin and ezetimibe, and ezetimibe was mixed with corresponding OPadry II White suspension is sprayed on the niacin tablet core with a barrier layer and film-coated.
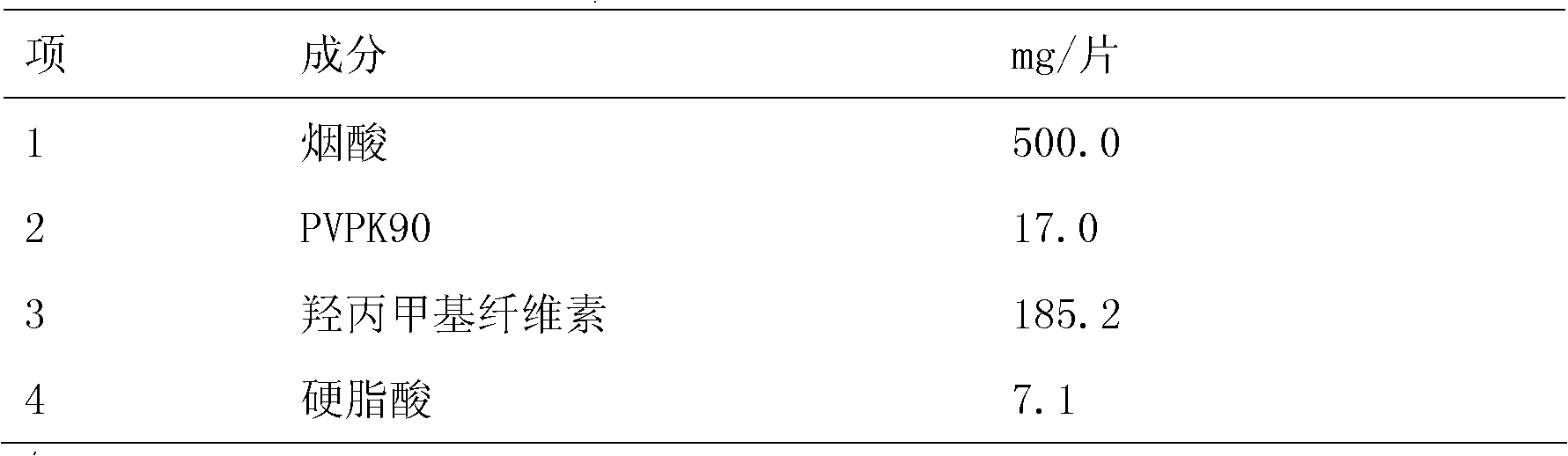
[0016] Preparation of Nicotinic Acid Tablets
[0017] Table 1
[0018]
[0019]
[0020] Process: Mix niacin, 57.5 grams of hypromellose, and PVP K90 evenly, make soft material with purified water, granulate, dry, granulate, add the remaining hypromellose and stearic acid, mix evenly, press piece.
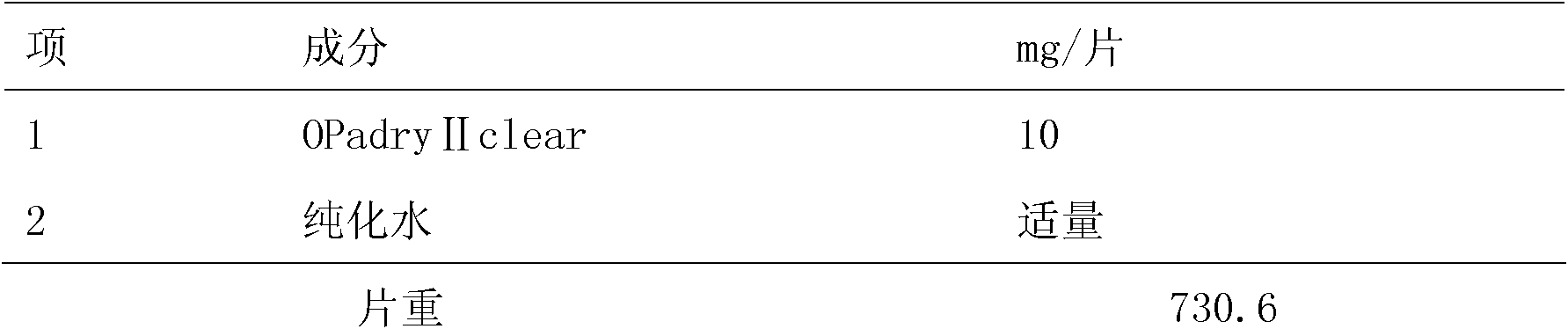
[0021] Preparation of isolation layer
[0022] Table 2
[0023]
[0024] Process: Disperse OPadry II clear coating powder in water and stir evenly; adjust the coating parameters, spray the coating powder suspension...
specific Embodiment 2
[0032] Specific Example 2 Ezetimibe / Etofibrate Composition
[0033] In this example, etofibrate and corresponding pharmaceutical excipients were made into pellets, sieved, and coated with an isolation coat to obtain etofibrate pellets; ezetimibe was mixed with corresponding pharmaceutical excipients to be evenly granulated, dried, Sieve to obtain ezetimibe granules; Etobet pellets, ezetimibe granules and corresponding pharmaceutical excipients are uniformly mixed, pressed into tablets, and coated.
[0034] Preparation of Etofibrate pellets
[0035]
[0036] Process: Mix powdered sugar and starch, add part of Etofibrate and mix evenly, dissolve shellac in ethanol to make it a 14.5% solution, granulate this solution to make the particle size between 125-800 μm, and obtain Etofibrate nuclear.
[0037] 2. Laminate Etobeta on the mother core, use 30kg of ethanol as a binder, make the particle size of the pellets 630-1250 μm, and finally coat with 40% shellac, and continuously ...
Embodiment 3
[0044] Embodiment 3: lipid-lowering effect experiment
[0045] Male Wistar rats (grade 2), weighing 160-170 g. Give high-fat diet 25g (high-fat diet formula: basic feed: cholesterol: lard: cholate: methylthiouracil = 8418: 4: 10: 1: 012) every day in a quantitative manner, and continue for 26 days to establish hyperlipidemia. mouse model.
[0046] In addition to a blank control group (group 0) that was not given high-fat feed, the experimental animals that successfully established the model were randomly divided into 9 groups, namely group A (model control); group B (ezetimibe) : dosage 10mg / time; group C (compound ezetimibe niacin); group D (compound acipimus ezetimibe); group E (compound nitrobetel ezetimibe); group F (compound ezetimibe fenofibrate ezetimibe); group G (compound bezafibrate ezetimibe); group H (compound etobate ezetimibe); group I (compound ciprofibrate ezetimibe) . All the drugs were suspended in 0.5% sodium carboxymethylcellulose and administered by in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com