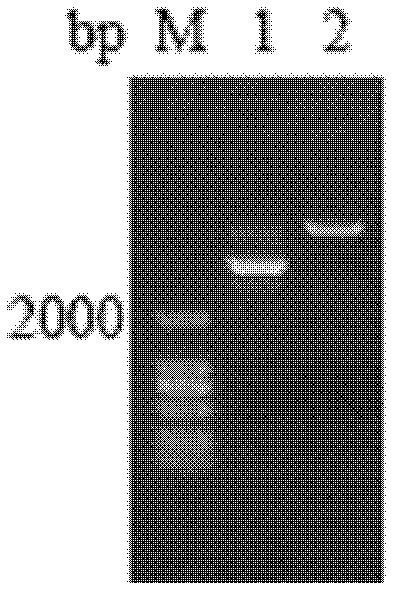Scylla paramamosain antiviral type lipopolysaccharide resistant factor as well as preparation method and application thereof
A technology of anti-lipopolysaccharide factor and pseudo-scylla, applied in antiviral agents, botany equipment and methods, biochemical equipment and methods, etc., can solve the problems of virus-free control methods, economic losses, etc., and achieve attractive applications Foreground, strong broad-spectrum antibacterial activity, effect of strong anti-WSSV activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Construction of Eukaryotic Recombinant Expression Vector for Antiviral Anti-lipopolysaccharide Factor Sp-ALF2 of Scylla pseudocarpus
[0072] According to the multiple cloning site of the pPIC9k vector, a specific upstream primer F1 and a downstream primer R1 were designed to amplify the ORF of the gene coding for Scylla sclerophyllus Sp-ALF2 (cDNA). A SnaB I restriction site and a base encoding His-tag were added to the 5' end of the upstream primer F1; a stop codon and an Avr II restriction site were added to the 5' end of the downstream primer R1. The upstream primer F1: 5′-CGTACGTACACCATCATCATCATCATCAGTATGAAGCTCTGGTAGC-3′, the downstream primer R1: 5′-AACCTAGGTC ACCCCTTCAGCCAGGCGGCC-3′, amplify the coding region fragment of Sp-ALF2. The PCR reaction conditions were: pre-denaturation at 94°C for 5 minutes; denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, extension at 72°C for 30 seconds, repeating 30 cycles; extension at 72°C for 10 mi...
Embodiment 2
[0075] Example 2 Induced expression of pPIC9k / Sp-ALF2 recombinant plasmid in Pichia pastoris GS115
[0076] The sequenced correct plasmid pPIC9k / Sp-ALF2 was linearized by Sal I digestion (see figure 2 ), transformed into Pichia pastoris GS115 competent cells by electric shock method, and induced expression.
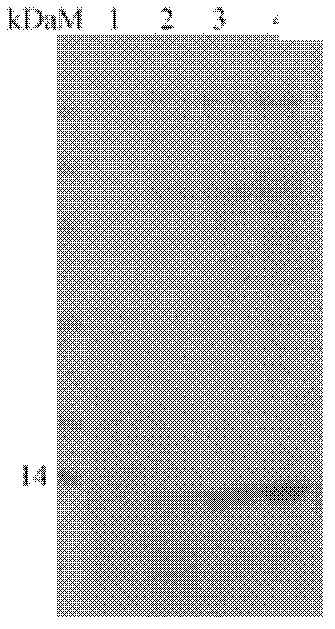
[0077] Pichia pastoris GS115 transformed with pPIC9k empty plasmid was used as the control, and Pichia pastoris GS115 transformed with pPIC9k / Sp-ALF2 recombinant plasmid was used as the experimental group. The expression of the target protein was detected by polyacrylamide gel electrophoresis (SDS-PAGE).
[0078] The results showed that Pichia pastoris GS115 transformed with the pPIC9k / Sp-ALF2 recombinant plasmid had significantly induced recombinant protein expression compared with before induction, and the protein band was around 14kDa (see image 3 ), which was similar to the calculated theoretical molecular weight, and was confirmed to be Sp-ALF2 after being identif...
Embodiment 3
[0079] Example 3 Purification, antiviral activity and antibacterial activity identification of pPIC9k / Sp-ALF2 recombinant plasmid induced expression product in Pichia pastoris GS115
[0080] 1. Purification of target protein by affinity chromatography
[0081] After a large amount of recombinant positive Pichia pastoris GS115 strain was induced and expressed, 1 L of the supernatant of the culture medium was collected by centrifugation to remove the bacterial cells, dialyzed twice with PBS (new dialysate was changed once every 12 hours), centrifuged at 12000 rpm for 35 minutes at 4°C, and the supernatant was obtained. Load the sample on the column. Then, the dialyzed protein was subjected to affinity chromatography using a metal chromatographic column. The elution peak of solution D was collected, and it was identified as Sp-ALF2 recombinant protein through SDS-PAGE electrophoresis analysis and mass spectrometry (see Figure 4 ).
[0082] 2. Anti-WSSV activity identification...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com