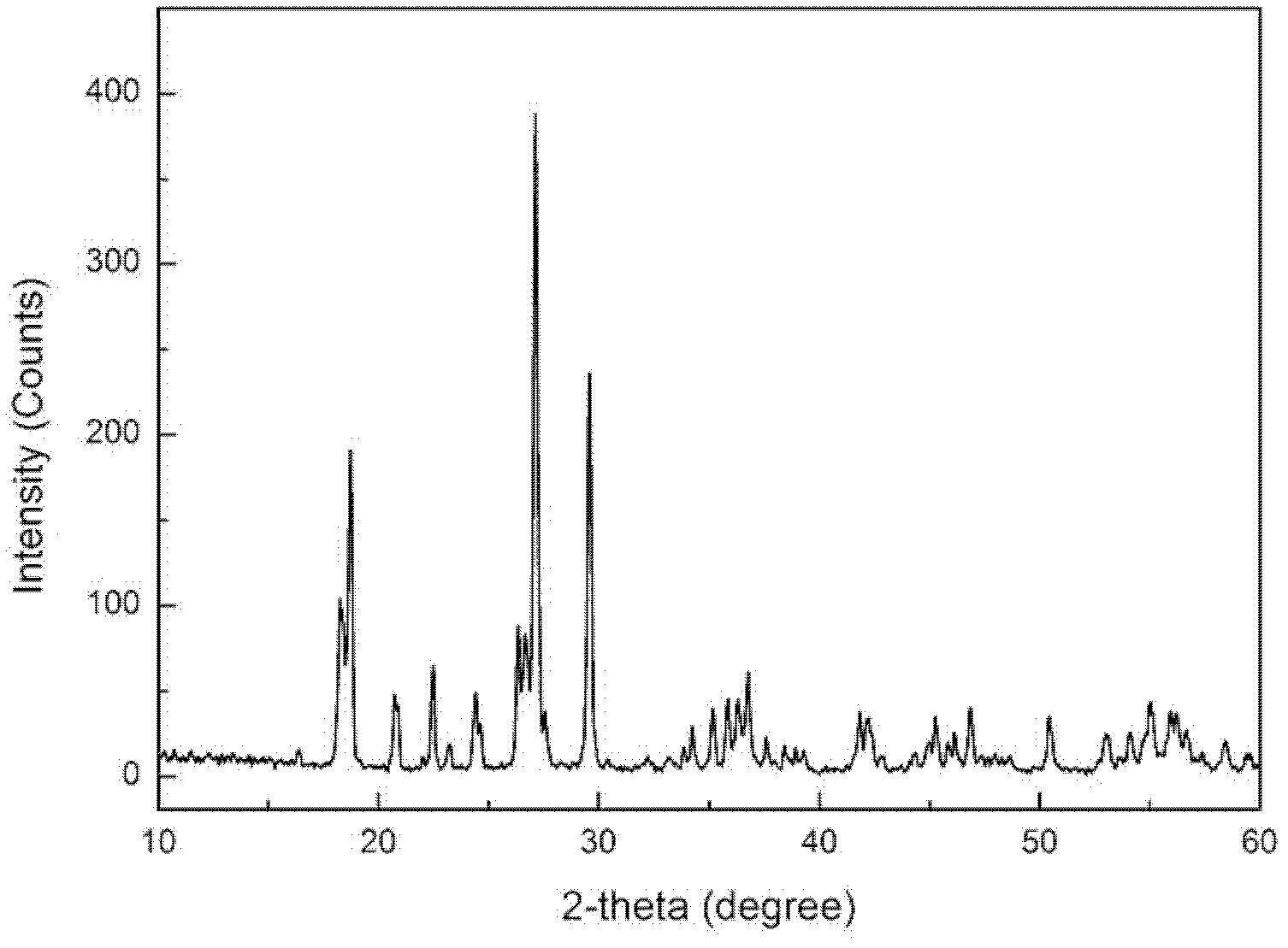
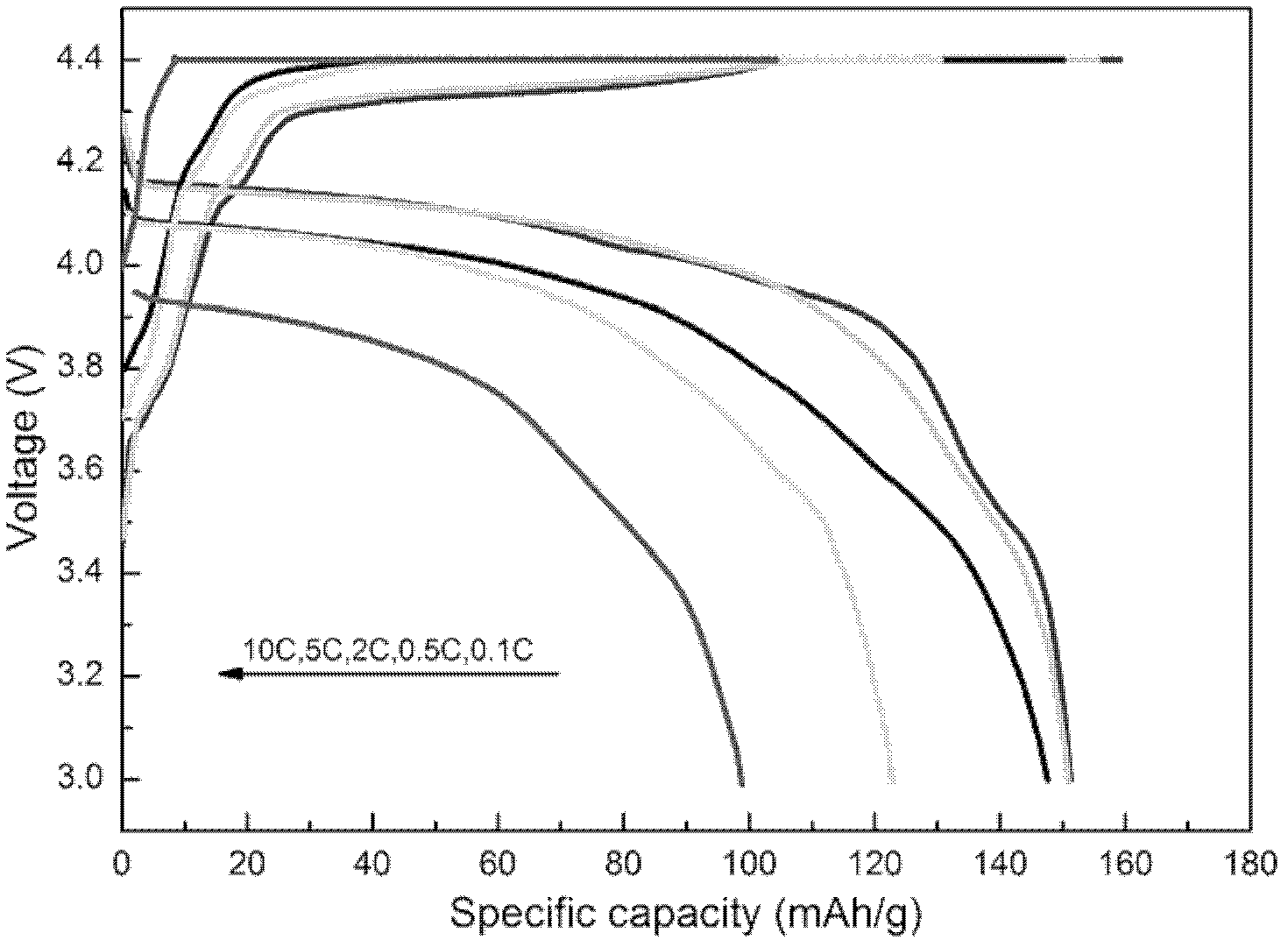
Method used for preparing lithium ion battery positive pole material fluophosphate vanadium lithium
A lithium-ion battery, lithium vanadium phosphate technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of uneven distribution of material components, unstable electrochemical performance, harsh control conditions, etc., to achieve excellent electrochemical performance , Excellent electrochemical performance and short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Using lithium fluoride, vanadium pentoxide and ammonium dihydrogen phosphate as raw materials, press LiVPO 4 According to the stoichiometric ratio of F, add oxalic acid (1.5 times the theoretical amount), mechanically activate for 20 hours until the pentavalent vanadium is completely reduced to trivalent vanadium, and then the resulting powder is stored at 600°C in an argon atmosphere. 650°C, 700°C, 750°C and 800°C for 12 hours to obtain the positive electrode material LiVPO 4 F, the resulting product was assembled into a button battery to measure its charge and discharge capacity and rate performance. Charge and discharge at different rates, the first discharge specific capacity is shown in Table 1.
[0029] Table 1 Experimental conditions and results of Example 1
[0030]
Embodiment 2
[0032] Using lithium carbonate, vanadium trifluoride, ammonium metavanadate and diammonium hydrogen phosphate as raw materials, according to the stoichiometric ratio of LiVPO4F, add ascorbic acid (adding 5 times the theoretical amount), and mechanically activate for 0.5 hours until the pentavalent vanadium is completely It is reduced to trivalent vanadium, and then the resulting powder is kept at 650°C in a nitrogen atmosphere for 0.5, 2, 6, and 12 hours to obtain the positive electrode material LiVPO 4 F, the obtained product was assembled into a button battery to measure its charge and discharge capacity and rate performance, and the charge and discharge were carried out at different rates, and the specific capacity of the first discharge is shown in Table 2.
[0033] Table 2 Experimental conditions and results of Example 2
[0034]
Embodiment 3
[0036] Using lithium hydrogen fluoride, vanadium dioxide and triammonium phosphate as raw materials, press LiVPO 4 According to the stoichiometric proportion of F, add hydrazine hydrate (according to 1 times the theoretical amount), mechanically activate for 6 hours until the pentavalent vanadium is completely reduced to trivalent vanadium, and then the obtained powder is kept at 600°C for 10 hours in a nitrogen atmosphere. Hours, the positive electrode material LiVPO 4 F, the obtained product was assembled into a button battery to measure its charge and discharge capacity and rate performance, and the charge and discharge were carried out at different rates, and the first discharge specific capacity at 0.1C, 0.5C, 2C, 5C and 10C rates was 150.7 respectively mAh·g -1 , 148.5mAh·g -1 , 141.3 mAh·g -1 , 120.3 mAh·g -1 and 101.3 mAh·g -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com