Synthesizing method for cephalosporin compound
A synthesis method and compound technology, applied in the direction of organic chemistry and the like, can solve problems such as unfavorable large-scale production, complex process operation, unfavorable environmental protection, etc., and achieve the effects of reducing production cost and environmental protection expenditure, saving solvent, and improving product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
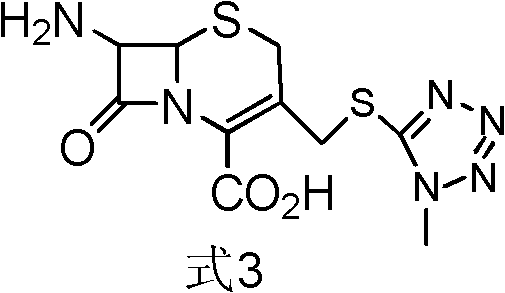
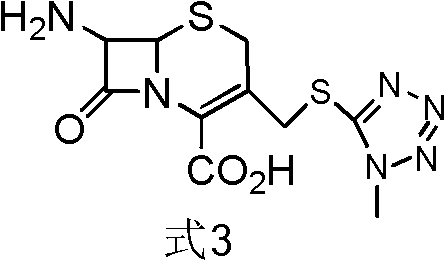
[0058] [Example 1] Preparation of formula 3 compound 7-amino-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid intermediate
[0059] Get the acetonitrile complex of 600g boron trifluoride (wherein BF Content 17%, be converted into 1.504mol), 45g methylmercaptotetrazolium (0.388mol), 100g 7-aminocephalosporanic acid (0.366mol) put reaction bottle , reacted at 30°C for 1 h, and added 100 g of N,N-dimethylacetamide to obtain an intermediate solution of the compound of formula 3.
Embodiment 2
[0060] [Example 2] Preparation of formula 3 compound 7-amino-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid intermediate
[0061] Get the dimethyl carbonate complex of 250g boron trifluoride (wherein BF3 content 40%, be converted into 1.475mol), 45g methylmercaptotetrazolium (0.388mol), 100g 7-aminocephalosporanic acid (0.366mol) , 400 g of dimethyl carbonate was placed in a reaction flask, reacted at 30°C for 1 h, and 100 g of N,N-dimethylformamide was added to obtain an intermediate solution of the compound of formula 3.
Embodiment 3
[0062] [Example 3] Preparation of formula 3 compound 7-amino-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid intermediate
[0063] Put 45g of methylmercaptotetrazolium (0.388mol), 100g of 7-aminocephalosporanic acid (0.366mol), and 400g of diethyl ether into a reaction bottle, and feed 150g of boron trifluoride (gas) (2.214mol), and react at 30°C for 1h 100 g of triethylamine was added to obtain an intermediate solution of the compound of formula 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com