Polymer anion exchange membrane and preparation method thereof
An anionic polymer and monomer technology, applied in the field of anion exchange membrane and its preparation, can solve the problems of poor chemical stability, achieve good thermal stability and alkali resistance, simple and safe preparation process, good mechanical properties and stable dimensions sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of monomers containing imidazole functional groups (Reference: Macromolecules 2010, 43, 10196-10200).
[0035] 1) Add 2,7-dibromo-9,9-bis(6'-bromohexyl)fluorene (5.0g, 7.7mmol), p-hydroxyphenylboronic acid (1.2g, 8.5mmol) to a three-necked flask, 40ml THF and 30ml 2M K 2 CO 3 Solution, nitrogen protection, plus catalyst PdCl 2 (dppf) 160mg, slowly raise the temperature to 70°C, and react for 24 hours. The crude product was extracted and purified by column chromatography to obtain 3.46 g of light yellow crystal 2,7-bis(4'-hydroxyphenyl)-9,9-bis(6'-bromohexane)fluorene, with a yield of 66.2%.
[0036] 2) Add 2,7-bis(4'-hydroxyphenyl)-9,9-bis(6'-bromohexane)fluorene (2.0g, 2.96mmol), 1,2-dimethyl Kimidazole (1ml, 12.5mmol), 20ml of acetonitrile, under nitrogen protection, reacted at 70°C for 12 hours. The crude product was washed with anhydrous ether and dichloromethane to obtain 2.45 g of light yellow solid with a yield of 95.3%.
[0037] The preparati...
Embodiment 2
[0039] Add 0.005mol to the three-necked flask 0.005mol 0.011mol Cs 2 CO 3 , 30ml dimethylsulfoxide and 15ml toluene. Under the protection of nitrogen, the temperature was raised to 120° C. for 4 hours, and the water generated during the reaction was removed, the temperature was slowly raised to 140° C., and the reaction was refluxed for 8 hours. The obtained polymer was precipitated with deionized water, washed with deionized water and ethanol several times, and dried for future use. After fully dissolving 0.1 g of the polymer obtained in N,N-dimethylformamide, the polymer solution was dropped onto a polytetrafluoroethylene plate and dried at 70°C for 2 hours to prepare a Br-type anion exchange membrane .
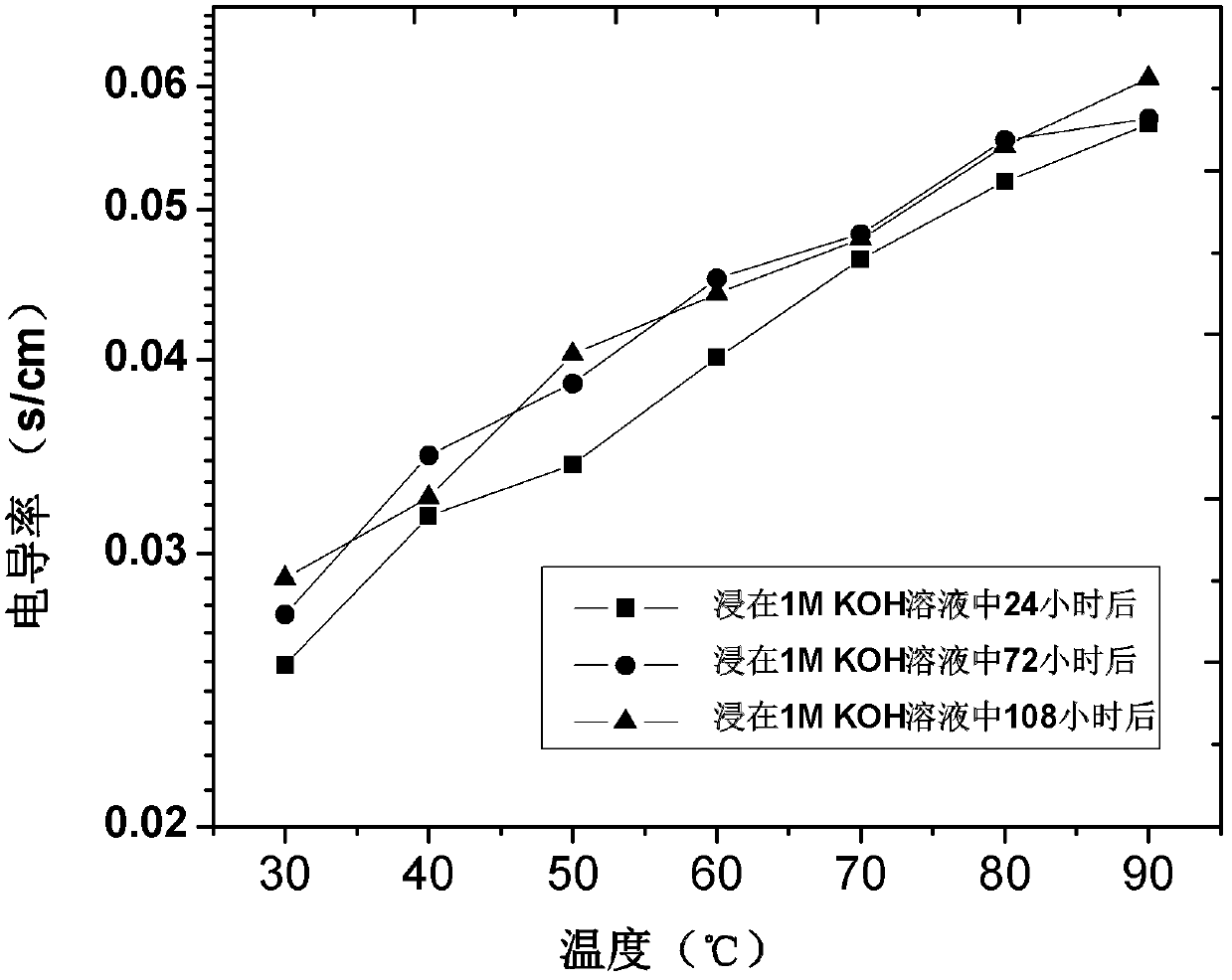
[0040]The resulting bromine-type anion-exchange membrane was placed in 1M KOH solution and soaked at 60°C for 24 hours until the Br - completely exchanged to OH - After that, get OH - type anion exchange membrane.
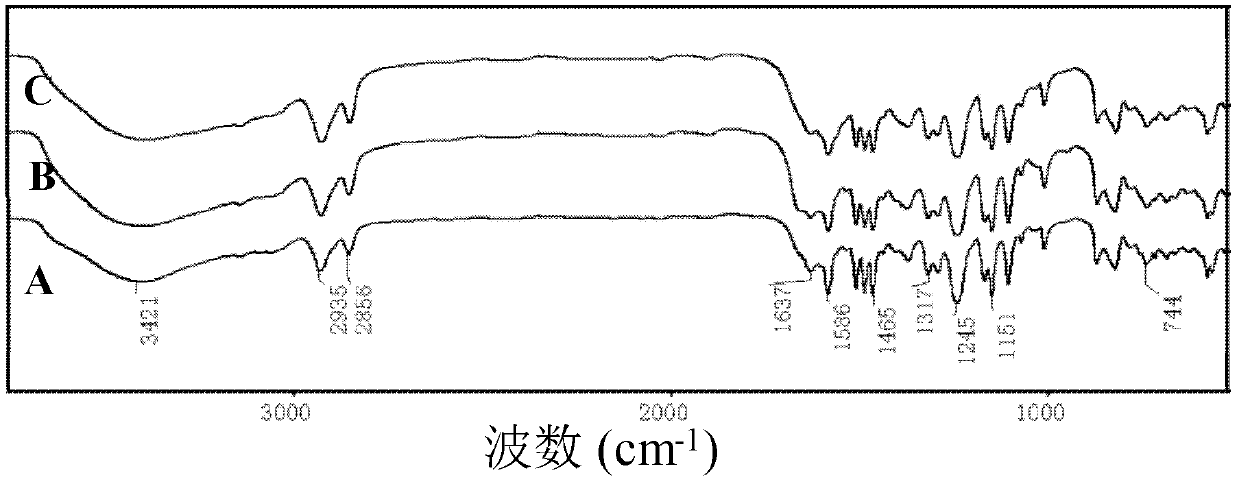
[0041] FT-IR Varian CP-3800spectrometer was use...
Embodiment 3
[0044] Add 0.005mol to the three-necked flask 0.005mol 0.011mol K 2 CO 3 , 30ml dimethylsulfoxide and 15ml toluene. Under the protection of nitrogen, the temperature was raised to 120°C for 4 hours, and the water generated during the reaction was removed, the temperature was slowly raised to 140°C, and the reaction was carried out under reflux for 16 hours. The obtained polymer was precipitated with deionized water, washed with deionized water and ethanol several times, and dried for future use. After fully dissolving 0.1 g of the obtained polymer in dimethyl sulfoxide, the polymer solution was dropped onto a polytetrafluoroethylene plate, and dried at 70° C. for 6 hours to prepare a Br-type anion exchange membrane.
[0045] The obtained bromine-type anion exchange membrane was placed in 1M KOH solution, soaked at 60°C for 24 hours, and the Br - completely exchanged to OH - Afterwards, residual KOH was removed with deionized water to obtain OH - type anion exchange me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com