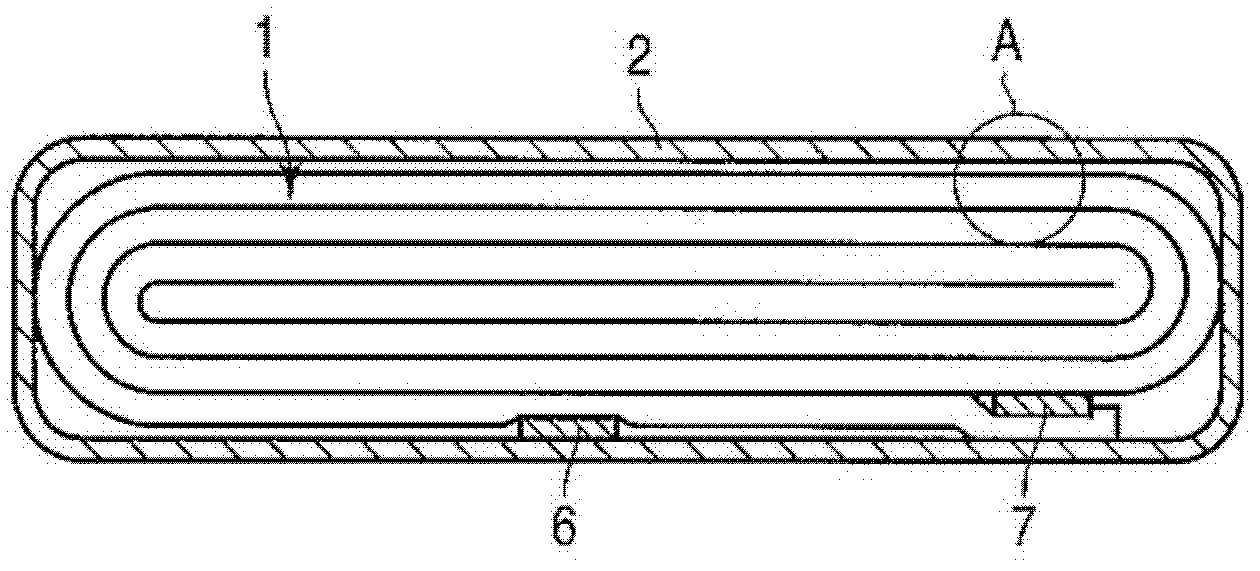
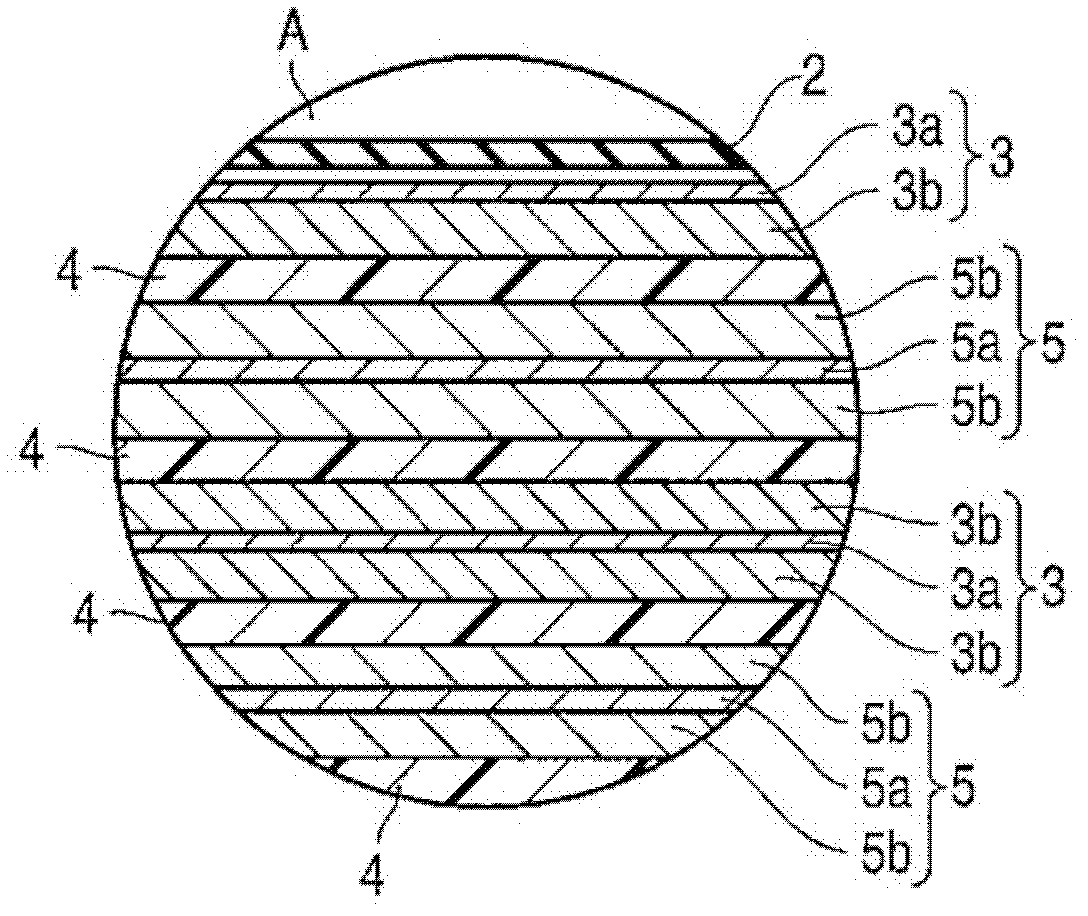
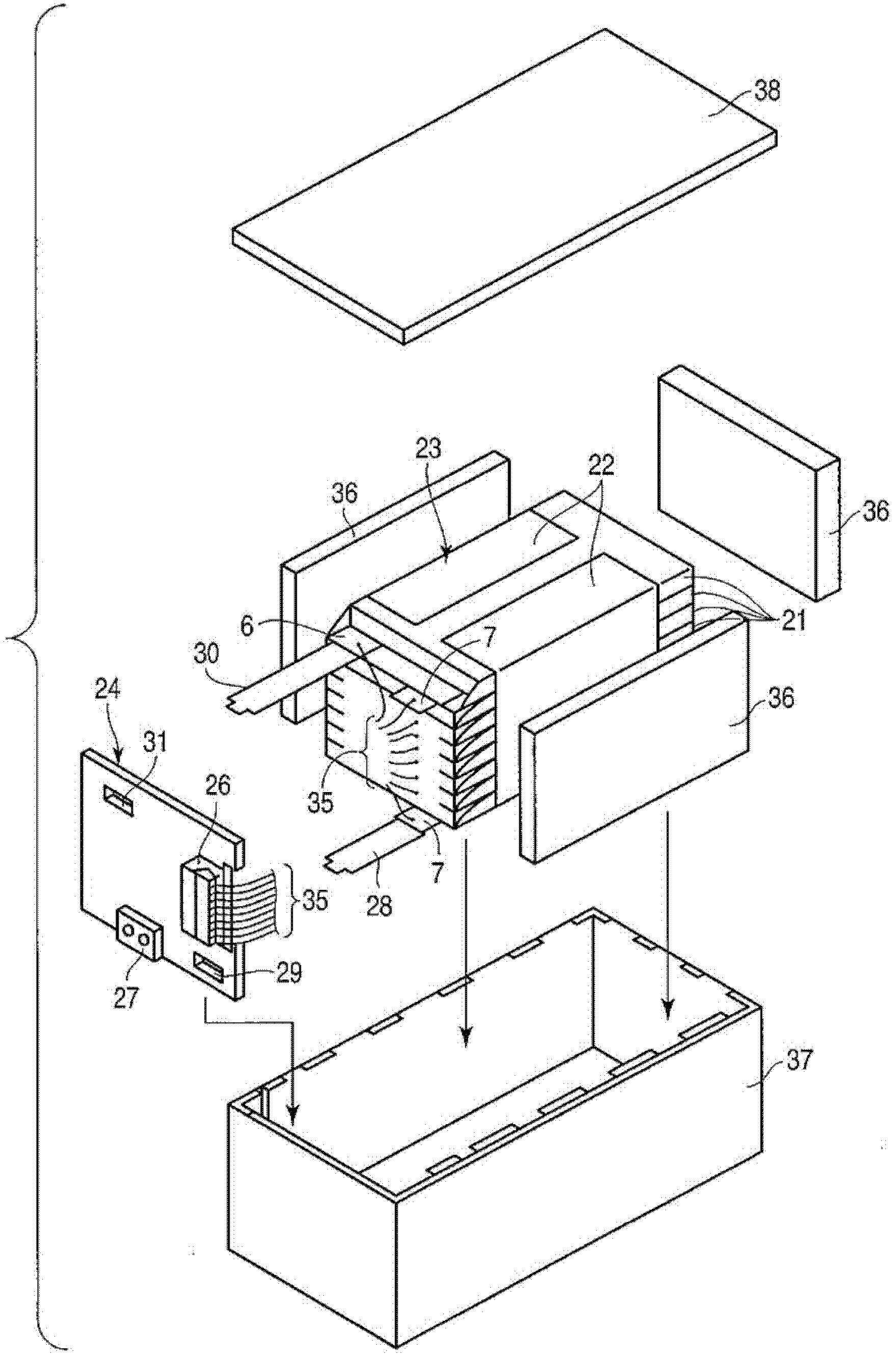
Active material for cell, nonaqueous electrolyte cell and cell pack
A non-aqueous electrolyte and active material technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of low Li ion diffusivity and less mobile Li ions, and achieve the effect of high capacity and excellent high current characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138]
[0139] First, N-methylpyrrolidone (NMP) was added with lithium nickel composite oxide (LiNi 0.82 Co 0.15 Al 0.03 O 2 ) powder 90 wt %, 5 wt % of acetylene black as a conductive agent, and 5 wt % of polyvinylidene fluoride (PVdF), these were mixed to prepare a slurry, and the slurry was applied to an aluminum foil with a thickness of 15 μm. Both sides of the current collector are then dried and pressurized, thereby making a positive electrode layer with a density of 3.15g / cm 3 the positive pole.
[0140]
[0141] First, potassium carbonate (K 2 CO 3 ) and anatase titanium oxide (TiO 2 ) were mixed and fired at 1000°C for 24 hours to synthesize K 2 Ti 4 O 9 . The K obtained by using zirconia microbeads 2 Ti 4 O 9 Dry pulverization was performed for about 3 hours to adjust the particle size, followed by washing with pure water to prepare a proton exchange precursor. The obtained proton exchange precursor was put into a hydrochloric acid solution having ...
Embodiment 2~5
[0152] A titanium composite oxide was synthesized by setting the pulverization time, proton exchange time, temperature and time of the first heat treatment, temperature increase rate, and temperature and time of the second heat treatment, and temperature increase rate to the conditions described in Table 1 below, respectively, A non-aqueous electrolyte secondary battery was assembled in the same manner as in Example 1, except that the obtained titanium composite oxide was used as the active material of the negative electrode.
[0153] In addition, in Examples 3 to 5, 3% by weight of sucrose was dissolved in a pure water / ethanol mixed solution to prepare a solution, the substance obtained by the first heat treatment was put into the solution, and the solvent was volatilized while stirring to prepare a solution. The carbon-forming precursor coating material is subjected to the second heat treatment.
Embodiment 6~12
[0162] The grinding time, the proton exchange time, and the time, temperature, and heating rate of the heat treatment (first heat treatment) were respectively set to the conditions described in the following Table 3 to synthesize a titanium composite oxide, and the obtained titanium composite oxide was used as A non-aqueous electrolyte secondary battery was assembled in the same manner as in Example 1 except for the active material of the negative electrode.
[0163] In addition, the crystallite grain sizes in the four crystal plane orientations of the titanium composite oxides obtained in Examples 2, 12 and Comparative Example 1 are shown in Image 6 middle.
[0164] The 0.2C discharge capacity and the 2C discharge capacity were measured for each of the batteries of Examples 6 to 12 in an environment of 25°C, and the ratio (%) of the 2C discharge capacity to the 0.2C discharge capacity was obtained as the capacity retention ratio (%). The results are shown in Table 4 below. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com