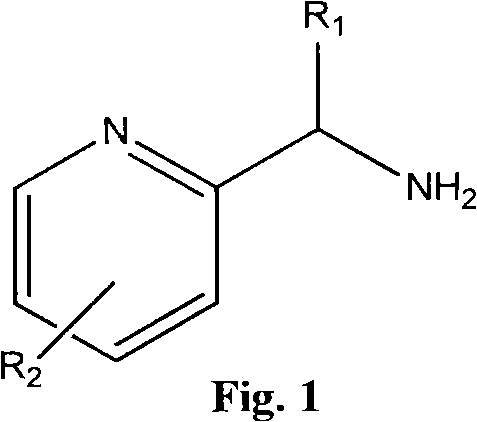
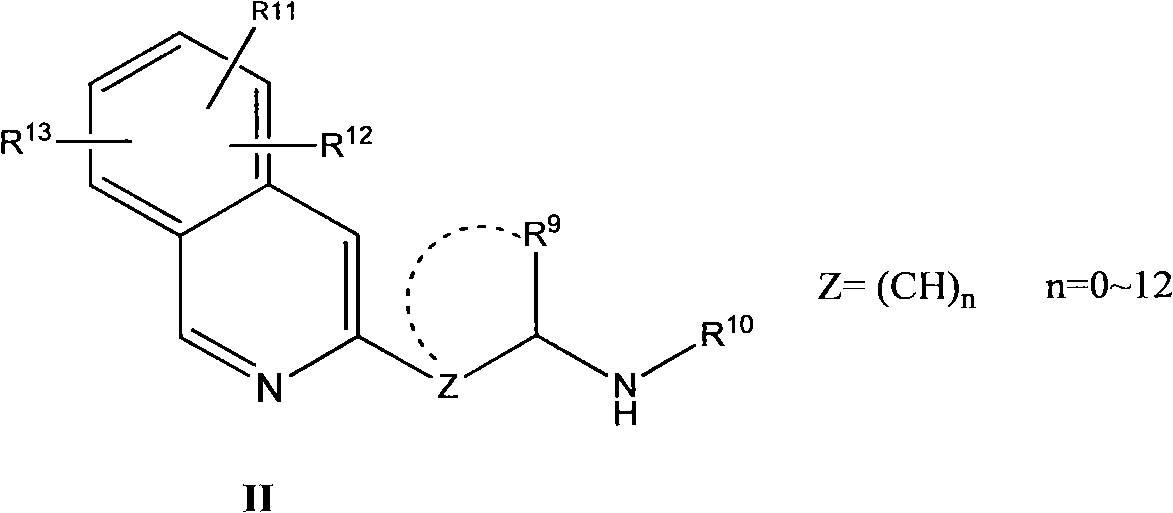
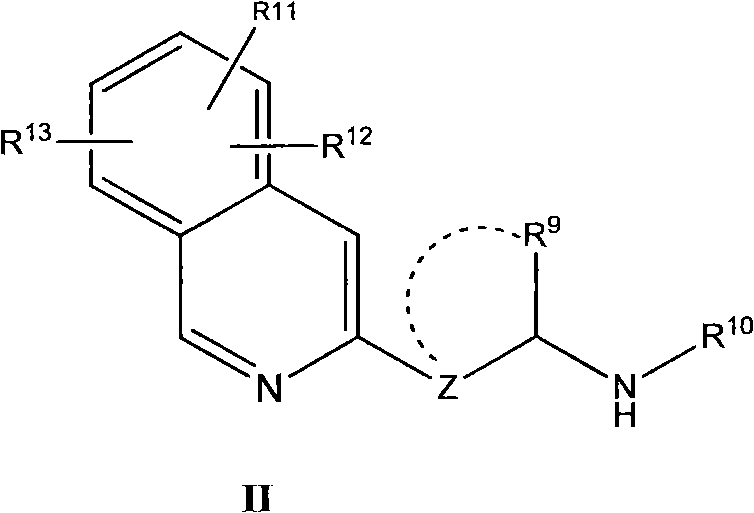
Nitrogen heterocycle ligand transition metal complex, and preparation and catalytic application thereof
A transition metal and nitrogen heterocyclic technology, applied in the field of nitrogen-containing heterocyclic ligands, can solve the problems of low selectivity and ineffective catalysis of catalysts, and achieve the effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of isoquinoline ammonia ligand
[0047]
[0048] Preparation of 2-aminomethylisoquinoline (7)
[0049] Add 2-cyanoisoquinoline (0.8 g) and 80 ml of methanol into the hydrogenation kettle, and stir to suspend 10% Pd / C (0.8 g). Displacing air, passing H 2 , pressurized to 5 atmospheres, and stirred for 16 hours. After the reaction was completed, filter with diatomaceous earth, and wash the filter cake with methanol. The resulting mother liquor was concentrated to dryness, then dissolved with 9 ml of absolute ethanol, saturated with hydrogen chloride gas, then cooled to 5°C, and a solid was precipitated, filtered, and dried to obtain 2-aminomethylisoquinoline (7) hydrochloride Salt 449 mg.
[0050] Preparation of 1-aminomethylisoquinoline (8)
[0051] 1-Aminomethylisoquinoline (8) can be prepared by the same process.
[0052]
Embodiment 2
[0053] The preparation of embodiment 2 isoquinoline ammonia ligand 14
[0054]
[0055]
[0056] Preparation of 2-acetylisoquinoline (11)
[0057] Intermediate 9 (800 mg) was placed in a 100 ml three-necked flask, and after replacing the air, 20 ml of dried THF was added, cooled to -78°C, and MeMgBr was slowly added. After the addition was completed, the temperature was slowly raised to -40°C, and the reaction was carried out for 100 minutes. After TLC monitors that the reaction is complete, the saturated ammonium chloride aqueous solution quenches the reaction, extracts with 250 milliliters of ethyl acetate, sherwood oil / ethyl acetate (10 / 1) passes through the column, and separates to obtain 2-acetyl isoquinoline (11), 502 mg white solid, yield 80%.
[0058] Preparation of (S)-1-(isoquinoline)ethanol (12)
[0059] Intermediate 11 (170 mg), RuCl2[(R)-xyl-Binap][(R)-Daipen] (1 mg), t-BuOK (6 mg) were added to the reaction kettle, after degassing, 3 ml of iso propanol....
Embodiment 3
[0064] Synthesis of the procatalyst of embodiment 3: RuCl 2 [(S)-BINAP]DMF 2
[0065]
[0066] [RuCl 2 (η 6 -benzene)] 2 (6.5 mg), 1 equivalent of (S)-BINAP (16.3 mg) dissolved in 2.0 mL of DMF, transferred to a 10 mL Schlenk tube under argon atmosphere and degassed. After the suspension was heated at 100°C for 30 minutes, it was cooled to room temperature, and the solvent was removed to obtain a solid product, which was dried under vacuum at 50°C for 2 hours.
[0067] RuCl 2 [(S)-binap] dmf 2 、RuCl 2 [(S)-Tolbinap] dmf 2 、RuCl 2 [(R)-xylbinap]dmf 2 、RuCl 2 [(S,S)-Skewphos]dmf 2 、RuCl 2 [(S)-phanephos]dmf 2 、RuCl 2 [(S,S)-Dipamp]dmf 2 、RuCl 2 [(S,S)-Chiraphos]dmf 2 、RuCl 2 [Mondyphos] dmf 2 、RuCl 2 [(S)-Segphos]dmf 2 、RuCl 2 [(S,S)-Diop]dmf 2 etc. can be prepared in a similar manner.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com