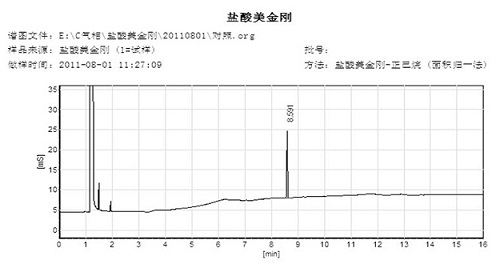
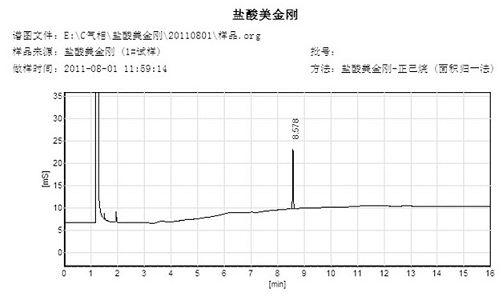
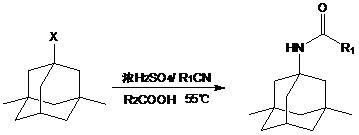Synthetic method of memantine hydrochloride
A technique for synthesizing memantine hydrochloride, which is applied in the amidation reaction and method improvement field of synthesizing memantine hydrochloride, can solve problems such as thick reaction mixture and difficult stirring, and achieve simple post-reaction treatment, shortened process time, and improved efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation of 1-bromo-3,5-dimethyladamantane
[0040] Take 200g of 1,3-dimethyladamantane in a three-necked bottle, connect the drying pipe and exhaust gas absorption device. Heated to 75°C, and 190ml of bromine was added dropwise. React for 26 hours and cool to room temperature. Add 400 ml of dichloromethane, and add saturated sodium bisulfite solution under ice cooling until the mixture turns light yellow. Static separation, the organic phase was obtained. The aqueous phase was extracted with dichloromethane (80ml×3), the organic phases were combined, washed with saturated sodium carbonate solution until neutral, and then washed with 100ml of saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated, and distilled under reduced pressure to collect 80 °C fraction, 275 g of colorless transparent liquid was obtained, with a yield of 92.9%.
[0041] (2) Preparation of 1-acetamido-3,5-dimethyladamantane
[0042] Take 31.5ml of acetonit...
Embodiment 2
[0046] (1) Preparation of 1-bromo-3,5-dimethyladamantane
[0047] With embodiment 1 (1).
[0048] (2) Preparation of 1-acetamido-3,5-dimethyladamantane
[0049] Take 32ml of acetonitrile and 5ml of glacial acetic acid, put them into a three-necked bottle, and add 30ml of concentrated sulfuric acid dropwise under cooling in an ice bath. Control the temperature of the mixture below 20°C. After the dropwise addition, 10 g of 1-bromo-3,5-dimethyladamantane (dissolved in 10 ml of glacial acetic acid) was added dropwise while controlling the internal temperature below 55°C. After the dropwise addition, it was heated to 55°C, the reaction solution changed from colorless to light yellow, and gradually turned into vermilion. After about 30 minutes, the color faded and the reaction solution turned into light yellow. The reaction was continued at 55°C for 24 hours with stirring. Cool to room temperature, pour into 200ml of water, and white crystals precipitate out. After suction fil...
Embodiment 3
[0053] (1) Preparation of 1-bromo-3,5-dimethyladamantane
[0054] With embodiment 1 (1).
[0055] (2) Preparation of 1-acetamido-3,5-dimethyladamantane
[0056] Take 300ml of acetonitrile and 380ml of glacial acetic acid, put them into a three-necked bottle, and add 260ml of concentrated sulfuric acid dropwise under ice cooling. Control the temperature of the mixture below 20°C. After the dropwise addition, 100.4 g of 1-bromo-3,5-dimethyladamantane (dissolved in 100 ml of glacial acetic acid) was added dropwise while controlling the internal temperature below 55°C. After the dropwise addition, it was heated to 55°C, the reaction solution changed from colorless to light yellow, and gradually turned into vermilion. After about 30 minutes, the color faded and the reaction solution turned into light yellow. The reaction was continued at 55° C. for 24 hours with stirring. Cool to room temperature, pour into 2000ml of water, and white crystals precipitate out. After suction fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com