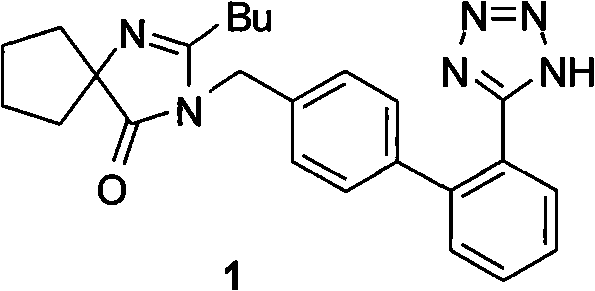
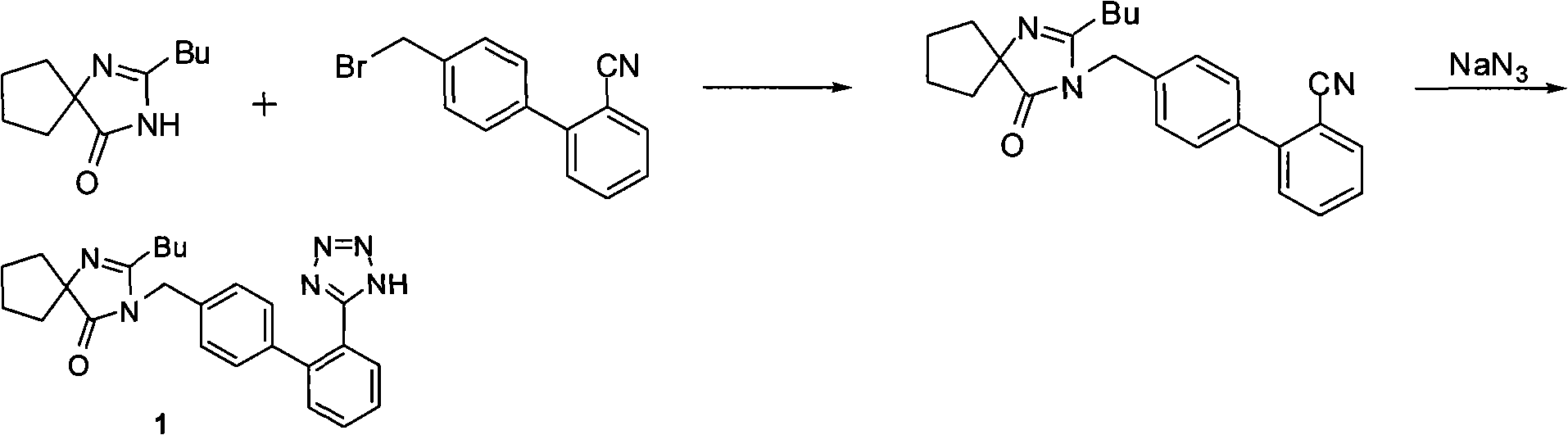
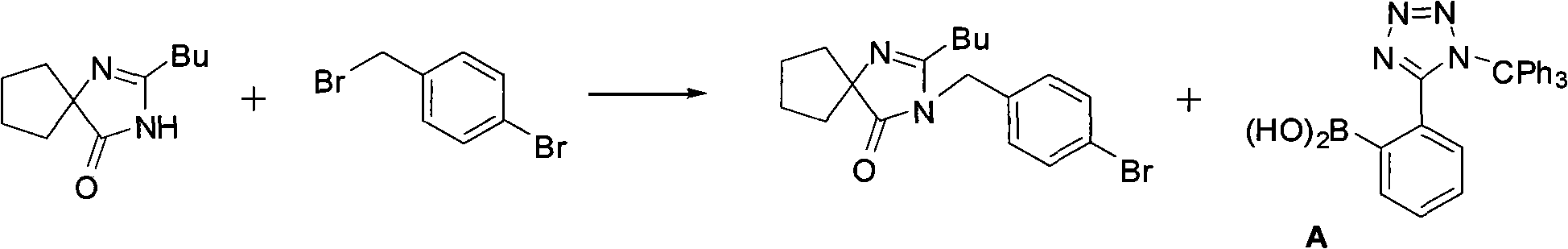
Method for synthesizing irbesartan and intermediate thereof
A technology of intermediates and compounds, which is applied in the field of drug preparation, can solve the problems of high cost, cumbersome preparation process, and safety risks, and achieve the effects of saving raw material costs, convenient synthesis methods, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Preparation of compound of formula 3: 1-aminocyclovaleronitrile oxalate
[0054] 20g (0.41mol) sodium cyanide dissolved in 40mL water, add 23g (0.43mol) ammonium chloride aqueous solution 60mL, 20% ammonia water 35mL and 30g (0.36mol) cyclopentanone methanol solution 40mL, stir at room temperature for 1.5 hours, warm up Stir for another 45 minutes at 60°C, stop heating, and continue stirring for 45 minutes. Reduce to room temperature, extract with 80mL×6 methylene chloride, dry the organic phase with anhydrous magnesium sulfate, filter, and concentrate the filtrate. The remaining oily substance 41g is dissolved in 100mL acetone, and 25g (0.28mol) oxalic acid in acetone solution 200mL is added with stirring. The solid was separated out, filtered, the filter cake was rinsed with acetone, and dried to obtain 50.0 g of white solid, yield 70.0%, Mp 220°C.
Embodiment 2
[0055] Example 2: Preparation of the compound of formula 4: 1-aminocyclopentaneamide
[0056] 50.0g (0.25mol) of compound (3) was slowly added to 75mL of concentrated sulfuric acid, stirred, bubbles were generated, and the temperature rose to 90°C. After adding, keep stirring for 1 hour, cool to 35℃, pour into ice water containing 300mL concentrated ammonia, extract 6 times with chloroform containing 10% methanol, combine the organic phases, dry with anhydrous magnesium sulfate and filter, and distill the filtrate under reduced pressure Solvent, white solid 35.0g, Mp 94~95℃.
Embodiment 3
[0057] Example 3: Preparation of compound of formula 5: 2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one hydrochloride 30.0g (0.23mol) compound (4), 33mL (0.24mol) Add 500mL of tetrahydrofuran to triethylamine, dropwise add 50mL of 30g (0.25mol) valeryl chloride solution in tetrahydrofuran, stir for 0.5 hour, add 70mL of water, 150mL of methanol and 70g of potassium hydroxide, reflux for 5 hours, and add 90g of chlorinated to room temperature Ammonium, stirred for 0.5 hours, concentrated under reduced pressure, added 40 mL of water to the residue, extracted with ethyl acetate 100 mL×3, combined the organic layers, added anhydrous magnesium sulfate, dried and filtered, added 20 mL of methanol to the filtrate, and cooled to below 20°C. Pass HCI gas to PH=1~2, cool to 0~5℃, stir for 1 hour, and filter to obtain 40.2g of white powdery solid, yield 88.4%, Mp 259~261℃.
[0058] IR(KBr, cm -1 ): 2962.1, 2787.6, 2631.8, 1777.9 (C=O), 1641.4, 1517.4, 1061.2. 1 H-NMR(DMSO-d 6 , 400MHz) δ: 13.648...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com