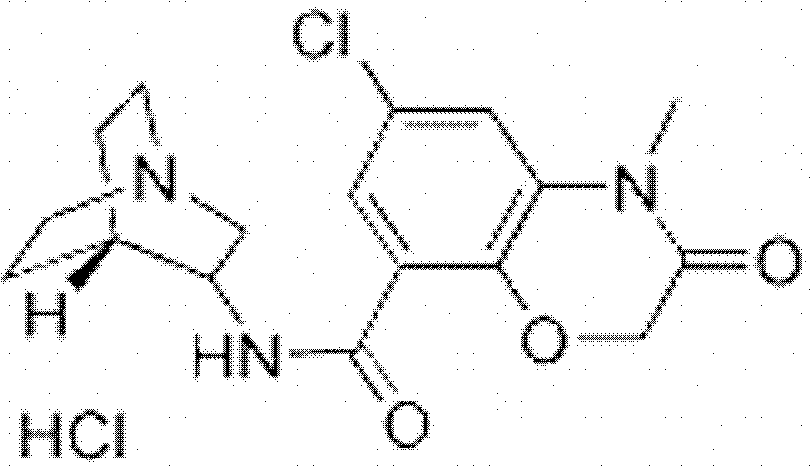
Azasetron hydrochloride injection and preparation method thereof
A technology of azasetron hydrochloride and azasetron, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of unstable aqueous solution, affecting product quality, and light Sensitivity and other issues to achieve the effect of reducing adverse reactions, overcoming instability, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of Azasetron Hydrochloride Injection: Weigh sodium chloride, thiourea and methionine respectively according to the batch production instructions, dissolve them in fresh and cooled water for injection in turn, stir and dissolve completely, then add to the instruction amount . Azasetron hydrochloride was weighed so that the weight ratio of azasetron, thiourea, methionine and sodium chloride was 20:3:6:36.
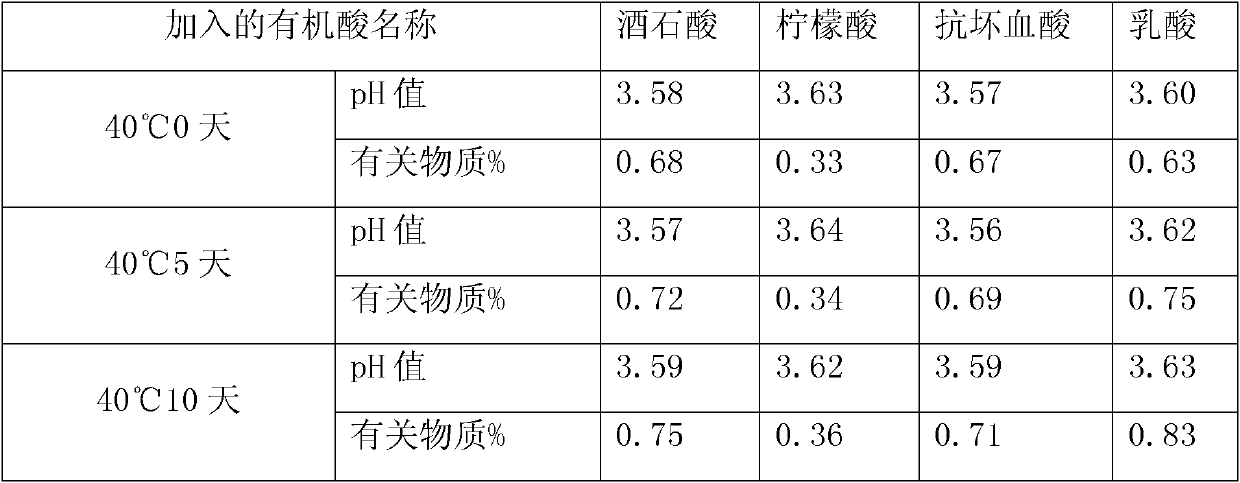
[0018] Add azasetron to the above solution, stir to dissolve, and the operation after adding azasetron hydrochloride needs to be protected from light. Adjust the pH to 3.5-4.5 with 0.1mol / L citric acid. The liquid medicine is filtered through a titanium rod filter and then poured into the fine filter tank. The medicinal liquid in the preparation tank is delivered to the fine filter tank through the infusion pump, and the medicinal liquid is filtered through a 0.45 μm cartridge filter, and the pH value and content are measured by sampling. Visible foreign...
Embodiment 2
[0022] This example detects the influence of different potting environments on the stability of azasetron hydrochloride. Experiments have proved that the use of linked line potting has a lower reject rate and higher yield than single-machine potting lamp inspection.
[0023] Potting environment
Embodiment 3
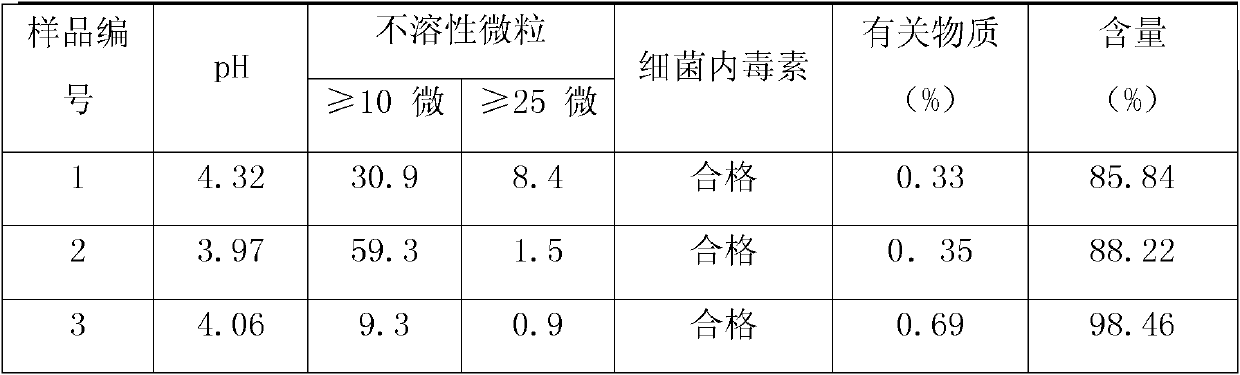
[0025] In this example, different antioxidants were added to the azasetron injection, and the contents of related substances were detected. The preparation method of azasetron injection is the same as that of Example 1. All the samples in this small test were filled and sealed with 1ml ampoule linkage line, and the number of each sample was 1000.
[0026] Sample number
antioxidant
pH
Sample No. 1
pH after preparation is 4.07
No. 2 sample
Methionine+sodium metabisulfite
pH after preparation is 4.08
Sample No. 3
Thiourea + Methionine + Sodium Metabisulfite
pH after preparation is 4.09
[0027] The results proved that when sodium metabisulfite was added to the injection, the content of related substances in azasetron hydrochloride injection would increase or even exceed the standard.
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com