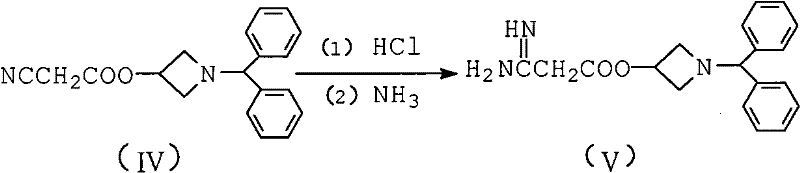Process for producing azelnidipine
A technology for the production of azhedipine, which is applied in the direction of organic chemistry, etc., to achieve the effects of mild reaction conditions, relatively high yield, and optimized feed ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
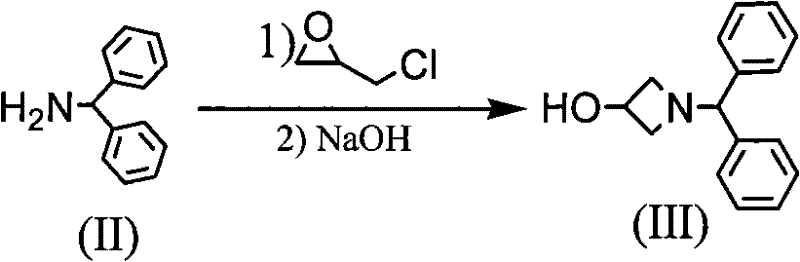
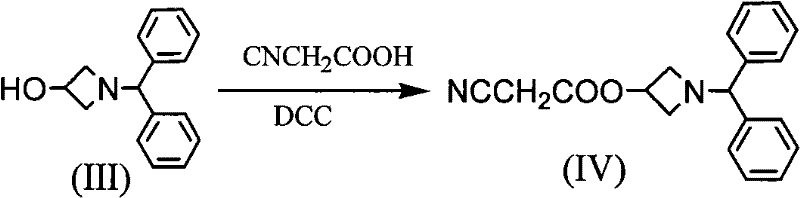
[0007] In the synthesis workshop, diphenylmethylamine is used as raw material to synthesize through addition, cyclization, esterification, acidification, ammoniation, condensation and other reactions, and the crude product of azedipine is refined, dried, mixed and packaged in a clean area to obtain azedipine. The specific reaction is as follows:
[0008] 1. Addition and ring closure reactions
[0009] Add methanol, diphenylmethylamine and epichlorohydrin into the reaction kettle, stir at room temperature for 24 hours, after the reaction is completed, heat up to reflux for 24 hours, cool, filter and collect the precipitated solid, then concentrate the mother liquor to recover the raw materials, continue heating and reflux for 18 hours, collect product, dichloromethane and H were added to the resulting solid 2 O, adjust the pH to 10-11 with 40% NaOH under stirring in an ice bath, let stand, separate the organic layer, dry over anhydrous magnesium sulfate, recover dichloromethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com