Conjugated block polymer, and its preparation method and application
A conjugated polymer and conjugated copolymer technology, which is used in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc. problems, to achieve the effect of improving solubility, improving crystallization properties, and reducing steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
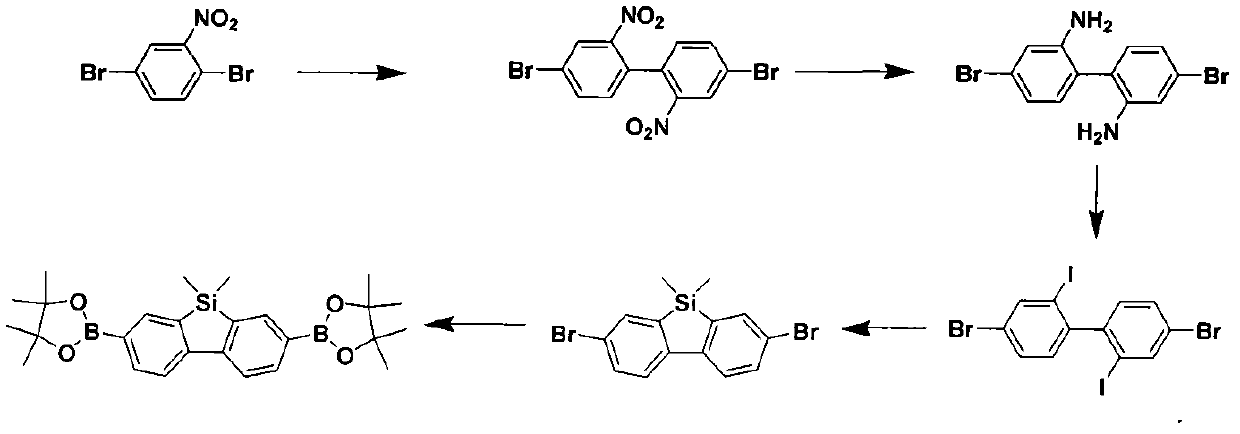
[0026] Example 1. Preparation of 9,9-dimethyl-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) dibenzo Silole
[0027] 9,9-Dimethyl-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)dibenzosilole is a polymer 1 is an important module in the synthesis, and its preparation reaction formula is as follows figure 1 shown.
[0028] The specific description is as follows:
[0029] Step 1) Mix 2,5-dibromonitrobenzene (50.0g) and copper powder (27.0g) in 200mL of N,N-dimethylformamide, heat the reaction at 125°C for 3 hours, and cool to room temperature. The remaining copper powder was removed by filtration, and the solvent in the filtrate was evaporated to dryness to obtain a crude product. The crude product was washed with 500 mL of methanol, then dissolved in toluene, filtered to remove inorganic salts, and the filtrate was evaporated to dryness to obtain yellow crystal 4,4'-dibromo-2,2'-dinitrobiphenyl (26 g, 84 %);
[0030] Step 2) Add 4,4'-dibromo-2,2'-dinitrobenzene ...
Embodiment 2
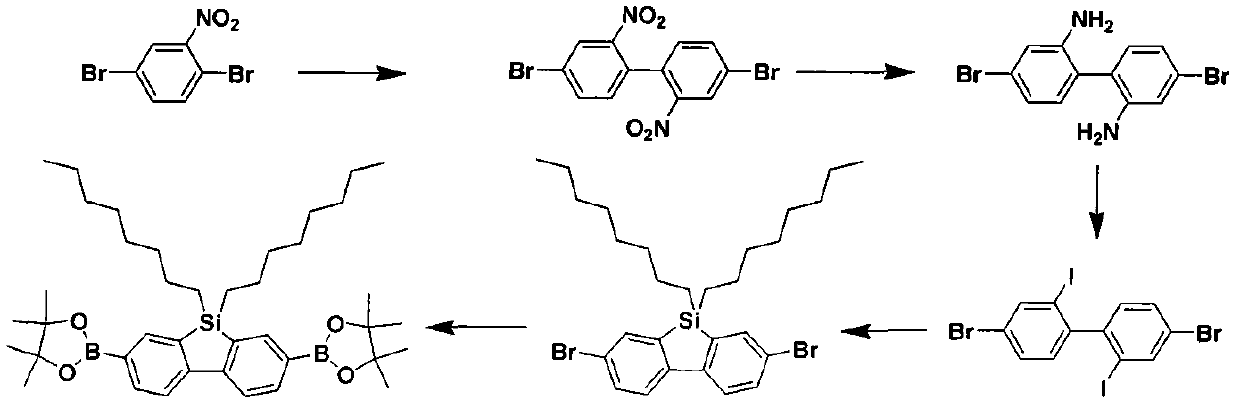
[0034] Example 2. Preparation of 9,9-dioctyl-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) dibenzo Silole
[0035] 9,9-dioctyl-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)dibenzosilole is a polymer 2 is an important module in the synthesis, and its preparation reaction formula is as follows figure 2 shown.
[0036] The specific description is as follows:
[0037]Synthesis of 9,9-dioctyl-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)dibenzosilole Among them, steps (1) to (3) are consistent with the steps in Example 1, see Example 1 for details;
[0038] Step 4) Add 4,4'-dibromo-2,2'-diiodobiphenyl (1.5g) into a single-necked bottle containing 50mL of tetrahydrofuran, and after repeated degassing, use a cold trap to adjust the solution temperature to -90°C. Add n-butyllithium (4.27mL) into it, stir at -90°C for 1 hour, then inject dioctyldichlorosilane (1.50mL) into it, let the reaction return to room temperature naturally, react overnight, and evapora...
Embodiment 3
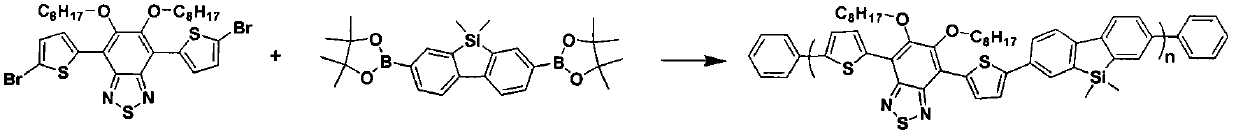
[0040] Embodiment 3, the block conjugated polymer 1 of preparation formula II structure
[0041] The conjugated polymer of formula II structure has 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolin-2-yl)-9,9-bis Methyldibenzosilole, 4,7-bis(5-bromothiophene-2-methylene)-5,6-dioctyloxy-1,3,5-benzothiadiazole Segment copolymer, its preparation reaction formula is as follows image 3 shown.
[0042] The specific description is as follows:
[0043] 1 mmol of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9,9-dimethyldibenzosilole , 1mmol of 4,7-bis(5-bromothiophene-2-methylene)-5,6-dioctyloxy-1,3,5-benzothiadiazole, 10mmol of sodium bicarbonate were added In the mixed solvent of 50ml of tetrahydrofuran, 10ml of toluene and 10ml of water, under the protection of nitrogen, add 0.1mmol of catalyst tetrakis (triphenylphosphine) palladium, heat and reflux for three days, cool to room temperature, and acetone settles after chloroform Three times, a block conjugated polymer 1 of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com