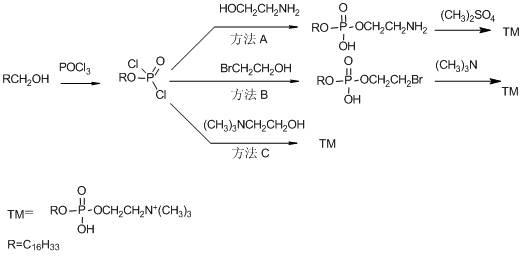
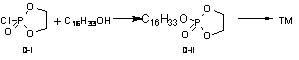Preparation method of phosphocholine alkyl ester
A technology of alkyl phosphate and choline, applied in the direction of phosphorus organic compounds, etc., can solve the problems of unstable intermediates, poor purity, difficult to purify, etc., and achieve the effects of simplified operation, improved purity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Step 1: Preparation of Intermediate B Hexadecyl Cyclic Phosphate Compound
[0048] In a 500ml four-necked flask, put 27.9g (1.1eq) of phosphorus oxychloride and 100ml of dry tetrahydrofuran into a 500ml four-necked flask, stir, and cool the reaction in an ice-salt bath to below -5°C. Ethylamine 25g (1.5eq) - tetrahydrofuran 100ml solution, add dropwise for about 2 hours, then continue to keep warm at -5°C for 0.5h, filter, and wash the filter cake with 20ml tetrahydrofuran;
[0049] Transfer the filtrate and washing liquid back to the four-necked flask, stir, cool to about -5°C, start to drop a solution of 10.3g (1eq) of ethylene glycol and 40g (2.4eq) of triethylamine - 40ml of tetrahydrofuran for about 1h After the addition is complete, react overnight, and the reaction is basically complete, filter, wash the filter cake with 40ml of tetrahydrofuran, and evaporate the filtrate to dryness under reduced pressure;
[0050] Add 250ml of dichloromethane to the obtained re...
Embodiment 2
[0058] Step 1: Preparation of Intermediate B Hexadecyl Cyclic Phosphate Compound
[0059] In a 500L reaction kettle, put 11.5kg (1.5eq) of phosphorus oxychloride and 50L of dry dioxane, stir, and cool the reaction in an ice-salt bath to below -10°C. At the same time, add 12kg (1eq) of cetyl alcohol dropwise. - Potassium carbonate 15.2kg (2.2eq) - dioxane 100L solution, about 2h to complete the dropwise addition, then continue to keep warm at -10°C for 0.5h, filter, and wash the filter cake with 3L dioxane;
[0060] The filtrate and lotion were transferred back to the four-necked bottle, stirred, cooled to about -10°C, and began to dropwise add a solution of 4.66kg (1eq) of ethylene glycol and 15.2kg (2.2eq) of potassium carbonate dissolved in 100L of dioxane, The dropwise addition was completed in about 1 hour, and the reaction was completed for 12 hours. The reaction was basically complete, filtered, and the filter cake was washed with 3 L of dioxane, and the filtrate was eva...
Embodiment 3
[0072] Step 1: Preparation of Intermediate B Hexadecyl Cyclic Phosphate Compound
[0073] In a 20L reaction kettle, put 0.75kg (0.8eq) of phosphorus oxychloride and 2L of dry acetone, stir, cool the reaction in an ice-salt bath to below -8°C, and drop 1.5kg (1eq) of cetyl alcohol-pyridine 0.7kg (1.4eq) - acetone 6L solution, about 1h to complete the dropwise addition, then continue to keep warm at -8°C for 0.5h, filter, and wash the filter cake with 3L of acetone;
[0074] Transfer the filtrate and lotion back to the four-necked bottle, stir, cool to about -8°C, start to add dropwise the solution of 0.8kg (2eq) of ethylene glycol and 1kg (2eq) of pyridine dissolved in 2L of acetone, and the dropwise addition is completed in about 1 hour. After 12 hours of reaction, the reaction was basically complete, filtered, the filter cake was washed with 0.5L acetone, and the filtrate was evaporated to dryness under reduced pressure;
[0075] Add 1 L of cyclohexanone to the obtained resi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com